EMA interacts with general practitioners, nurses, hospital and community pharmacists, specialist doctors and representatives of learned societies.
Healthcare professionals are involved as either representatives of European Union (EU) healthcare professionals' organisations, representatives of their own organisations or as individual experts, depending on the nature of the activity.
| Healthcare professionals representing their community | Healthcare professionals representing their organisations | Healthcare professionals as individual experts |
|---|---|---|
|
|
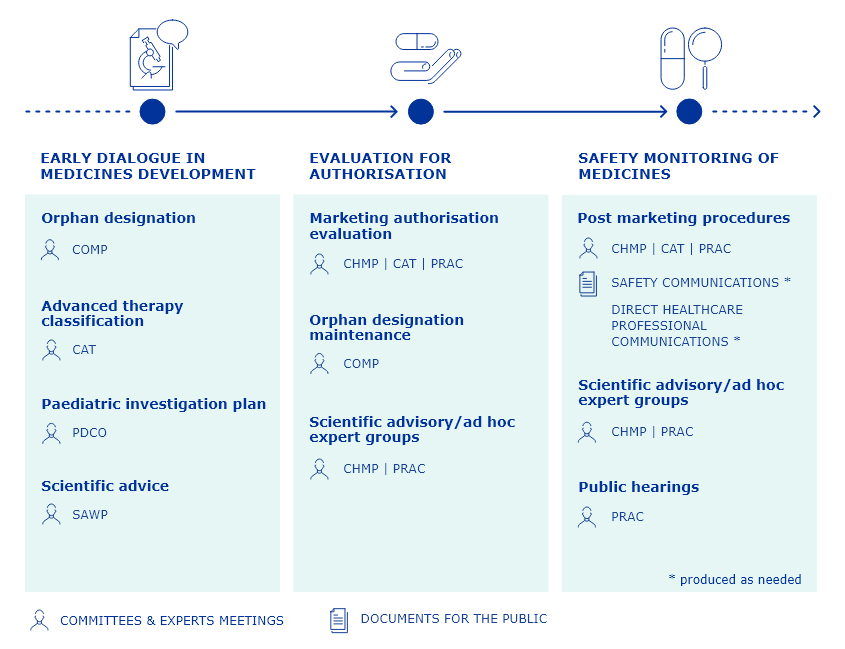
Overview of healthcare professionals' involvement along the medicines lifecycle at EMA
Healthcare professionals can get involved in every aspect of the regulatory lifecycle of a medicine from pre-submission and evaluation through to post-authorisation.
Getting involved by representing an organisation
Any not-for-profit organisation that fulfils the following eligibility criteria is welcome to express its interest in getting involved in the work of EMA:
- legitimacy, with statutes registered in the EU;
- clear mission and objectives with an interest in medicines;
- being representative of healthcare professionals throughout the EU;
- adequate structure and consultation modalities;
- transparency.
For full details, see:
- Criteria to be fulfilled by patient, consumer and healthcare professional organisations involved in European Medicines Agency (EMA) activities
- Assessment of patient, consumer and healthcare professional organisations’ compliance with EMA eligibility criteria
Interested organisations should fill in the following application form and send it to EMA together with any supporting information. The Agency welcomes applications at any time and will send an acknowledgment of receipt after receiving the application:
EMA will inform all organisations of the outcome of the evaluation of their application. EMA publishes information on eligible organisations on Eligible healthcare professionals' organisations.
Eligibility is re-assessed annually. For more information, see;
Getting involved as an individual expert
Individual healthcare professionals, as specialists in their area, contribute their clinical expertise directly into scientific regulatory discussions. EMA contacts experts primarily via its network of eligible organisations. However, individuals interested in working with EMA can also be included in the individuals’ stakeholder database, by completing the online registration form at:
For more information on the database, see:
Steps to take before getting involved
All experts, including healthcare professional representatives, need to register in EMA's experts management tool as ‘medicines experts’ before taking part in EMA activities, particularly related to medicine assessment.
EMA has a robust competing interests policy to ensure experts have no interests in the pharmaceutical or medical device industries that could affect their impartiality.
All healthcare professional representatives working with EMA must complete a publicdeclaration of interests and confidentiality undertaking form.
EMA will provide all new experts with instructions on how to:
- Get an EMA account to access the experts management tool
- Complete the registration process
When using the experts management tool, guidance is also available under the 'Useful information' tab.
