The Fee Regulation (Council Regulation (EC) No 297/95 of 10 February 1995 on fees payable to the European Agency for the Evaluation of Medicinal Products), and the Pharmacovigilance Fee Regulation (Regulation (EU) No 658/2014) determine EMA's fees.
Please note the regulation is changing from 1 January 2025
REGULATION (EU) 2024/568 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL on fees and charges payable to the European Medicines Agency, (the New Fee Regulation) has been published in the EU Official Journal following final adoption by the European Parliament and the Council. The Regulation will become applicable as of 1 January 2025. For more information, see European Commission: Regulation (EU) 2024/568 of the European Parliament and of the Council of 7 February 2024 on fees and charges payable to the European Medicines Agency.
Types of pharmacovigilance fees
The Agency charges two types of fees for pharmacovigilance activities:
- procedure based fees for:
- single assessment of periodic safety update reports (PSURs);
- assessment of post-authorisation-safety-study (PASS) protocols and study results;
- pharmacovigilance-related referrals.
The Agency has been charging for these procedures as of August 2014. The fees cover remuneration of national competent authorities for the scientific assessments rapporteurs carry out for the Pharmacovigilance Risk Assessment Committee.
- an annual fee, charged for nationally authorised medicines only. This fee is due on 1 July every year as of 2015. It supports the implementation and maintenance of measures from the 2010 pharmacovigilance legislation, including:
- literature monitoring;
- the European medicines web portal;
- enhanced functionalities for EudraVigilance;
- a repository of PSURs.
These activities directly benefit marketing-authorisation holders by reducing administrative burden, simplifying reporting and streamlining processes.
Calculating the fee
The basis of calculation of pharmacovigilance fees for PSURs, pharmacovigilance referrals and the annual fee is the chargeable unit. This is defined in Article 2 of Regulation (EC) 658/2014.
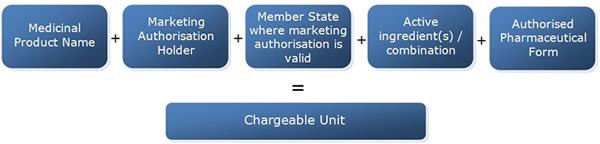
The chargeable unit is a unique combination of the following dataset derived from information on all medicines authorised in the European Union held by the Agency:
- Name of the medicinal product, as defined in point 20 of Article 1 of Directive 2001/83/EC;
- marketing authorisation holder;
- the Member State in which the marketing authorisation is valid;
- active substance or a combination of active substances;
- pharmaceutical form.
This is consistent with the obligation of marketing-authorisation holders referred to in points (b) and (c) of Article 57(2) of Regulation (EC) No 726/2004 to submit such information to the database referred to in point (l) of the second subparagraph of Article 57(1) of that regulation.
For guidance on how 'chargeable units' are derived from medicinal product information held within the Article 57 database, refer to:
- Calculating 'chargeable units' for pharmacovigilance fees as specified in Regulation (EU) No 658/2014: guidance on how 'chargeable units' are derived from medicinal product information held within the 'Article 57' databa...
Please note that, for PASS procedures, a flat amount to be paid in two instalments is applied.
Fee reductions and exemptions are available for micro-, small- and medium-sized-enterprises (SMEs) and for certain categories of medicines such as generics, well-established use, homeopathic and herbal products.
For full details, see:
- Explanatory note on pharmacovigilance fees payable to EMA (as of 4 October 2023) (as of 4 October 2023)
Advice notes
Prior to issuing an invoice, the Agency provides marketing-authorisation holders with an opportunity to review the information recorded in the Article 57 database by supplying the qualified person for pharmacovigilance with an advice note.
The advice note contains the line listing of the chargeable units and a reference to the related products (as recorded in the Article 57 database) as well as regulatory background and electronic submission guidance.
Paying your fees
For information and guidance on invoicing, terms and conditions of payment and how to set up a customer account with the Agency, see How to pay.
For information on general fees charged for services for obtaining and maintaining a marketing authorisation, see Fees payable to EMA.
Pharmacovigilance fees overview
EMA has prepared a series of short videos to provide further information on the fees it charges for pharmcavogilance activities. Topics covered include:
- Overview of pharmacovigilance fees
- Introducing the 'chargeable unit'
- Advice notes
- Fee reductions and fee exemptions
- Financial matters
Questions and answers
If you have a query related to pharmacovigilance fees, please visit the Pharmacovigilance fees: questions and answers page.