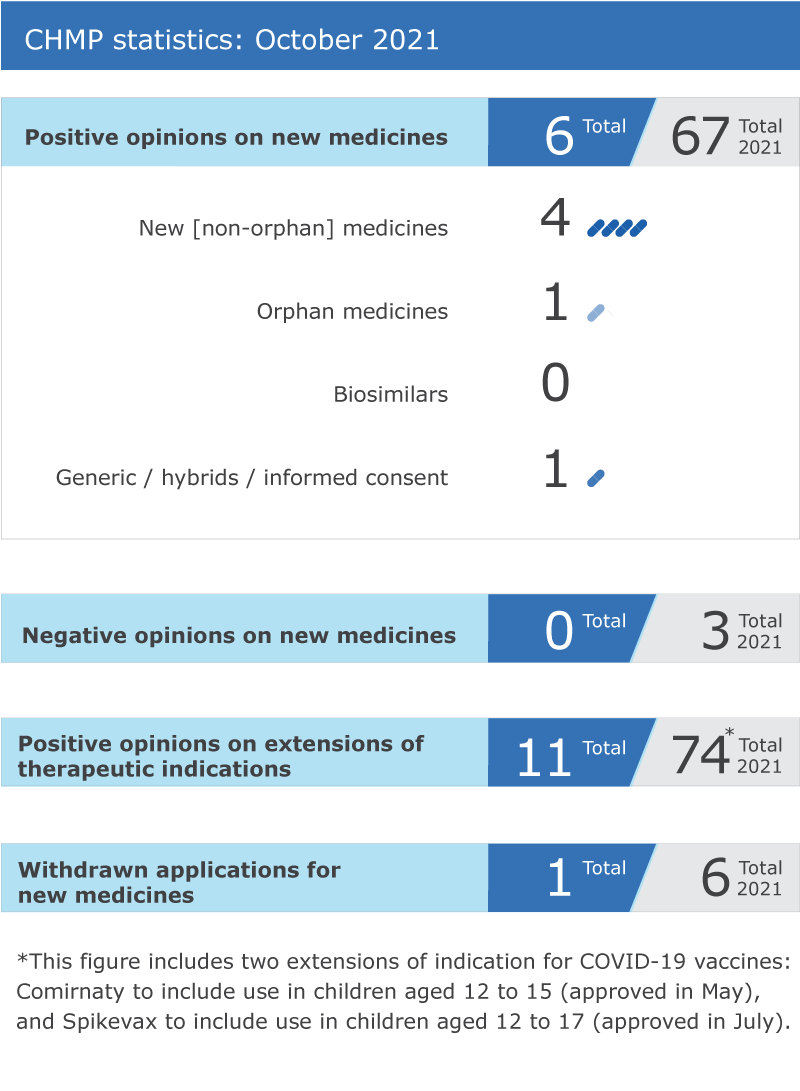
Six new medicines recommended for approval
EMA’s human medicines committee (CHMP) recommended six medicines for approval at its October 2021 meeting.
Trodelvy (sacituzumab govitecan) was granted a positive opinion for the treatment of unresectable or metastatic triple-negative breast cancer. See more details in the news announcement in the grid below.
The Committee adopted a positive opinion recommending the granting of a conditional marketing authorisation for Rybrevant (amivantamab) intended for the treatment of non-small cell lung cancer.
The Committee adopted a positive opinion for Aspaveli*(pegcetacoplan) for the treatment of adult patients with paroxysmal nocturnal haemoglobinuria.
The CHMP recommended granting a marketing authorisation for Cibinqo (abrocitinib) for the treatment of atopic dermatitis.
The Committee adopted a positive opinion for Vaxneuvance (pneumococcal polysaccharide conjugate vaccine (15-valent, adsorbed)), intended for prophylaxis against pneumococcal pneumonia and associated invasive disease.
One generic medicine, Sitagliptin SUN (sitagliptin fumarate), was granted a positive opinion by the CHMP for the treatment of type 2 diabetes.
Recommendations on extensions of therapeutic indication for ten medicines
The Committee recommended extensions of indication for Edistride, Forxiga, Hizentra, Kisplyx, Lenvima, Repatha, Skyrizi, Xeljanz, Zeposia and two extensions of indicationfor Keytruda.
Withdrawal of application
An application for an initial marketing authorisation for Zynyz (retifanlimab) was withdrawn. Zynyz was intended for the treatment of squamous carcinoma of the anal canal, a cancer of the tissues of the anus.
A question-and-answer document on the withdrawal is available in the grid below.
Negative outcome of Article 29 referral on Lidocain/Prilocain Idetec and associated names
The CHMP recommended the refusal of a marketing authorisation for Lidocain/Prilocain Idetec and associated names (lidocaine/prilocaine cream). Lidocain/Prilocain Idetec was intended to be applied to the skin and genital area to prevent pain during minor surgical or medical procedures, and for the treatment of leg ulcers.
For more information on this negative opinion, see the question-and-answer document in the grid below.
Re-election of Bruno Sepodes as CHMP vice-chair
The CHMP re-elected Bruno Sepodes as its vice-chair for a second three-year term, starting in October 2021.
Agenda and minutes
The agenda of the October 2021 CHMP meeting is published on EMA's website. Minutes of the September 2021 CHMP meeting will be published in the coming weeks.
CHMP statistics
Key figures from the October 2021 CHMP meeting are represented in the graphic below.
*This product was designated as an orphan medicine during its development. Orphan designations are reviewed by EMA's Committee for Orphan Medicinal Products (COMP) at the time of approval to determine whether the information available to date allows maintaining the medicine’s orphan status and granting the medicine ten years of market exclusivity.
Positive recommendations on new medicines
| Name of medicine | Aspaveli |
| International non-proprietary name (INN) | pegcetacoplan |
| Marketing-authorisation applicant | Swedish Orphan Biovitrum AB (publ) |
| Therapeutic indication | Treatment of adult patients with paroxysmal nocturnal haemoglobinuria |
| More information | Aspaveli: Pending EC decision |
| Name of medicine | Cibinqo |
| INN | abrocitinib |
| Marketing-authorisation applicant | Pfizer Europe MA EEIG |
| Therapeutic indication | Treatment of atopic dermatitis |
| More information | Cibinqo: Pending EC decision |
| Name of medicine | Rybrevant |
| INN | amivantamab |
| Marketing-authorisation applicant | Janssen-Cilag International N.V. |
| Therapeutic indication | Treatment of non-small cell lung cancer |
| More information | Rybrevant: Pending EC decision |
| Name of medicine | Trodelvy |
| INN | sacituzumab govitecan |
| Marketing-authorisation applicant | Gilead Sciences Ireland UC |
| Therapeutic indication | Treatment of unresectable or metastatic triple-negative breast cancer |
| More information | News:First-in-class medicine to treat aggressive form of breast cancer |
| Name of medicine | Vaxneuvance |
| INN | pneumococcal polysaccharide conjugate vaccine (adsorbed) |
| Marketing-authorisation applicant | Merck Sharp & Dohme B.V. |
| Therapeutic indication | Prophylaxis against pneumococcal pneumonia and associated invasive disease |
| More information | Vaxneuvance: Pending EC decision |
Positive recommendation on new generic medicine
| Name of medicine | Sitagliptin SUN |
| INN | sitagliptin fumarate |
| Marketing-authorisation applicant | Sun Pharmaceutical Industries Europe B.V. |
| Therapeutic indication | Treatment of type 2 diabetes |
| More information | Sitagliptin SUN: Pending EC decision |
Positive recommendations on extensions of indications
| Name of medicine | Edistride |
| INN | dapagliflozin |
| Marketing-authorisation holder | AstraZeneca AB |
| More information | Edistride: Pending EC decision |
| Name of medicine | Forxiga |
| INN | dapagliflozin |
| Marketing-authorisation holder | AstraZeneca AB |
| More information | Forxiga: Pending EC decision |
| Name of medicine | Hizentra |
| INN | human normal immunoglobulin |
| Marketing-authorisation holder | CSL Behring GmbH |
| More information | Hizentra: Pending EC decision |
| Name of medicine | Keytruda |
| INN | pembrolizumab |
| Marketing-authorisation holder | Merck Sharp & Dohme B.V. |
| More information | Keytruda: Pending EC decision |
| Name of medicine | Kisplyx |
| INN | lenvatinib |
| Marketing-authorisation holder | Eisai GmbH |
| More information | Kisplyx: Pending EC decision |
| Name of medicine | Lenvima |
| INN | lenvatinib |
| Marketing-authorisation holder | Eisai GmbH |
| More information | Lenvima: Pending EC decision |
| Name of medicine | Repatha |
| INN | evolocumab |
| Marketing-authorisation holder | Amgen Europe B.V. |
| More information | Repatha: Pending EC decision |
| Name of medicine | Skyrizi |
| INN | risankizumab |
| Marketing-authorisation holder | AbbVie Deutschland GmbH & Co. KG |
| More information | Skyrizi: Pending EC decision |
| Name of medicine | Xeljanz |
| INN | tofacitinib |
| Marketing-authorisation holder | Pfizer Europe MA EEIG |
| More information | Xeljanz: Pending EC decision |
| Name of medicine | Zeposia |
| INN | ozanimod |
| Marketing-authorisation holder | Bristol-Myers Squibb Pharma EEIG |
| More information | Zeposia: Pending EC decision |
Referral under Article 29
| Name of medicine | Lidocain/Prilocain Idetec and associated names |
| INN | lidocaine/prilocaine cream |
| Applicant | International Drug Development France |
| More information | Lidocain/Prilocain Idetec and associated names: Questions and answers |
| Name of medicine | Zynyz |
| INN | retifanlimab |
| Marketing-authorisation applicant | Incyte Biosciences Distribution B.V. |
| More information | Zynyz: Withdrawn application |