This page provides an overview of the opinions adopted at the March 2014 meeting of the Committee for Medicinal Products for Human Use (CHMP) and other important outcomes.
Nine new medicines recommended for approval
The CHMP has recommended granting a marketing authorisation for Vynfinit (vintafolide) for the treatment of women with a sub-type of platinum-resistant ovarian cancer for which there are limited approved treatment options. The CHMP has recommended approval for Vynfinit together with the approval of two companion diagnostic medicines, Folcepri (etarfolatide) and Neocepri (folic acid) that will help identify patients who may benefit from treatment with Vynfinit. All three of these medicines have an orphan designation and were recommended for conditional marketing authorisations. Please see the press release in the grid below for more details.
The CHMP has recommended the granting of a marketing authorisation for Sylvant (siltuximab), a medicine for the treatment of adult patients with multicentric Castleman's disease. Sylvant has an orphan designation and was evaluated by accelerated assessment. Please see the press release in the grid below for more information.
The CHMP also gave a positive recommendation for Entyvio (vedolizumab) for the treatment of ulcerative colitis and Crohn's disease. Please see the press release in the grid below for more details.
The Committee also recommended the granting of a marketing authorisation for Jardiance (empagliflozin) for the treatment of type 2 diabetes.
Olysio (simeprevir) was recommended for marketing authorisation for the treatment of chronic hepatitis C in adult patients in combination with other medicinal products.
Revinty Ellipta (fluticasone furoate /vilanterol trifenatate) for the treatment of asthma and chronic obstructive pulmonary disease (COPD) was also recommended for approval by the Committee. This product was submitted as an informed consent application.
The generic medicine Ebilfumin (oseltamivir) also received a positive opinion from the CHMP for the prevention and treatment of influenza. Ebilfumin is a generic of Tamiflu.
Three recommendations on extensions of therapeutic indications
The CHMP recommended extensions of indications for Pegasys, Tresiba and Victoza.
Outcome of safety review
The CHMP concluded its review of the safety of propylene glycol in intravenous formulations for short-term use in children. The assessment report for this review will be published next week.
Recommendation on seasonal influenza vaccine for 2014-1015
The CHMP adopted EU-wide recommendations for the influenza virus strains that should be included in vaccines for the prevention of seasonal influenza next winter. For more information, please click here.
Agenda and minutes
The agenda of the March 2014 meeting is published on the EMA website. The minutes of the meeting will be published during the week following the April CHMP meeting. Minutes of the February 2014 CHMP meeting will be published next week.
CHMP statistics
Key figures from the March 2014 CHMP meeting are represented in the graphic below.
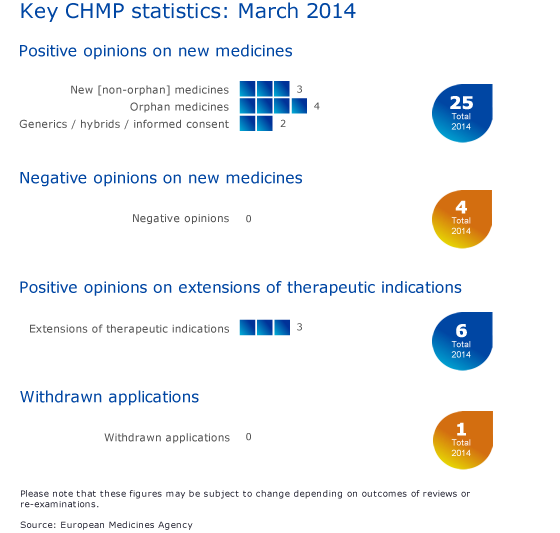
Positive recommendations on new medicines
| Name of medicine | Entyvio |
|---|---|
| International non-proprietary name (INN) | vedolizumab |
| Marketing-authorisation applicant | Takeda Pharma A/S |
| Therapeutic indication | Treatment of ulcerative colitis and Crohn's disease |
| More information | CHMP summary of positive opinion for Entyvio Press release: European Medicines Agency recommends approval of a locally targeted treatment for ulcerative colitis and Crohn's disease |
| Name of medicine | Folcepri |
|---|---|
| INN | etarfolatide |
| Marketing-authorisation applicant | Endocyte Europe, B.V |
| Therapeutic indication | Indicated for single photon emission computed tomography (SPECT) imaging |
| More information | CHMP summary of positive opinion for Folcepri Press release: European Medicines Agency recommends approval of new treatment for platinum-resistant ovarian cancer together with companion diagnostic |
| Name of medicine | Jardiance |
|---|---|
| INN | empagliflozin |
| Marketing-authorisation applicant | Boehringer Ingelheim International GmbH |
| Therapeutic indication | Treatment of type II diabetes mellitus |
| More information | CHMP summary of positive opinion for Jardiance |
| Name of medicine | Neocepri |
|---|---|
| INN | folic acid |
| Marketing-authorisation applicant | Endocyte Europe, B.V. |
| Therapeutic indication | Indicated for the enhancement of 99mTc-etarfolatide single photon emission computed tomography (SPECT) image quality |
| More information | CHMP summary of positive opinion for Neocepri Press release: European Medicines Agency recommends approval of new treatment for platinum-resistant ovarian cancer together with companion diagnostic |
| Name of medicine | Olysio |
|---|---|
| INN | simeprevir |
| Marketing-authorisation applicant | Janssen-Cilag International N.V. |
| Therapeutic indication | Indicated in combination with other medicinal products for the treatment of chronic hepatitis C in adult patients |
| More information | CHMP summary of positive opinion for Olysio |
| Name of medicine | Vynfinit |
|---|---|
| INN | vintafolide |
| Marketing-authorisation applicant | Endocyte Europe, B.V. |
| Therapeutic indication | Treatment of platinum resistant ovarian cancer |
| More information | CHMP summary of positive opinion for Vynfinit Press release: European Medicines Agency recommends approval of new treatment for platinum-resistant ovarian cancer together with companion diagnostic |
| Name of medicine | Sylvant |
|---|---|
| INN | siltuximab |
| Marketing-authorisation applicant | Janssen-Cilag International N.V. |
| Therapeutic indication | Treatment of multicentric Castleman's disease |
| More information | CHMP summary of positive opinion for Sylvant Press release: European medicines Agency recommends authorisation of first medicine for Castleman's disease |
Positive recommendation on new informed-consent application
| Name of medicine | Revinty Ellipta |
|---|---|
| INN | fluticasone furoate / vilanterol trifenatate |
| Marketing-authorisation applicant | Glaxo Group Ltd |
| Therapeutic indication | Treatment of asthma and chronic obstructive pulmonary disease |
| More information | CHMP summary of positive opinion for Revinty Ellipta |
Positive recommendation on new generic medicine
| Name of medicine | Ebilfumin |
|---|---|
| INN | oseltamivir |
| Marketing-authorisation applicant | Actavis Group PTC ehf |
| Therapeutic indication | Treatment and prevention of influenza |
| More information | CHMP summary of positive opinion for Ebilfumin |
Positive recommendations on extensions of therapeutic indications
| Name of medicine | Pegasys |
|---|---|
| INN | peginterferon alfa-2a |
| Marketing-authorisation holder | Roche Registration Limited |
| More information | CHMP post-authorisation summary of positive opinion for Pegasys |
| Name of medicine | Tresiba |
|---|---|
| INN | insulin degludec |
| Marketing-authorisation holder | Novo Nordisk A/S |
| More information | CHMP post-authorisation summary of positive opinion for Tresiba |
| Name of medicine | Victoza |
|---|---|
| INN | liraglutide |
| Marketing-authorisation holder | Novo Nordisk A/S |
| More information | CHMP post-authorisation summary of positive opinion for Victoza |
Re-examination of initial recommendation for marketing authorisation
| Name of medicine | Masican |
|---|---|
| INN | masitinib |
| Marketing-authorisation holder | AB Science |
| More information | Questions and answers on refusal of the marketing authorisation for Masican |