Five new medicines recommended for approval
EMA’s human medicines committee (CHMP) recommended five medicines for approval at its July 2019 meeting.
The Committee recommended granting a conditional marketing authorisation for Vitrakvi (larotrectinib), the first ‘histology-independent’ treatment in the European Union for solid tumours with a neurotrophic tyrosine receptor kinase (NTRK) gene fusion. NTRK gene fusions occur very frequently in a number of rare cancers. For more information, please see the press release in the grid below.
The CHMP granted a positive opinion for Epidyolex* (cannabidiol) for the treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome, two forms of epilepsy. Epidyolex contains an active substance derived from cannabis and is the first to receive a positive opinion in the EU centralised procedure.
Inbrija (levodopa) received a positive opinion for the treatment of symptoms of ‘off’ periods in Parkinson’s disease.
The Committee adopted a positive opinion for Trogarzo (ibalizumab), for the treatment of HIV infection.
The CHMP recommended for approval the generic medicine Deferasirox Mylan (deferasirox), for the treatment of chronic iron overload due to blood transfusions in patients with beta thalassaemia major, non-transfusion-dependent thalassaemia syndromes and other anaemias.
Start of re-examination of recommendation for new medicine
The applicant for Evenity (romosozumab) has requested a re-examination of the Committee's negative opinion for this medicine adopted at the June 2019 meeting. The CHMP will re-examine the opinion and issue a final recommendation. For more information on this negative opinion, please see the question-and-answer document in the grid below.
Eight recommendations on extensions of therapeutic indication
The Committee recommended extensions of indication for Empliciti, Keytruda, Lonsurf, Lucentis, Soliris, Stelara, Tecentriq and Zerbaxa.
Start of re-examination of recommendations on extension of therapeutic indications
The applicants for Revolade (eltrombopag) and Translarna (ataluren) have requested re-examination of the Committee's negative opinions for these medicines adopted at the June 2019 meeting. The CHMP will re-examine the opinions and issue final recommendations. For more information on these negative opinions, please see the question-and-answer documents in the grid below.
Updated restrictions for Gilenya
The CHMP recommended that the multiple sclerosis medicine Gilenya (fingolimod) must not be used in pregnant women and in women able to have children who are not using effective contraception. If a woman becomes pregnant while using Gilenya, the medicine must be stopped and the pregnancy will have to be closely monitored. This is because the active substance in Gilenya, fingolimod, can harm the unborn baby and may cause birth defects. For more information, please see the public health recommendation in the grid below.
Agenda and minutes
The agenda of the July 2019 meeting is published on EMA's website. Minutes of the June 2019 CHMP meeting will be published in the coming weeks.
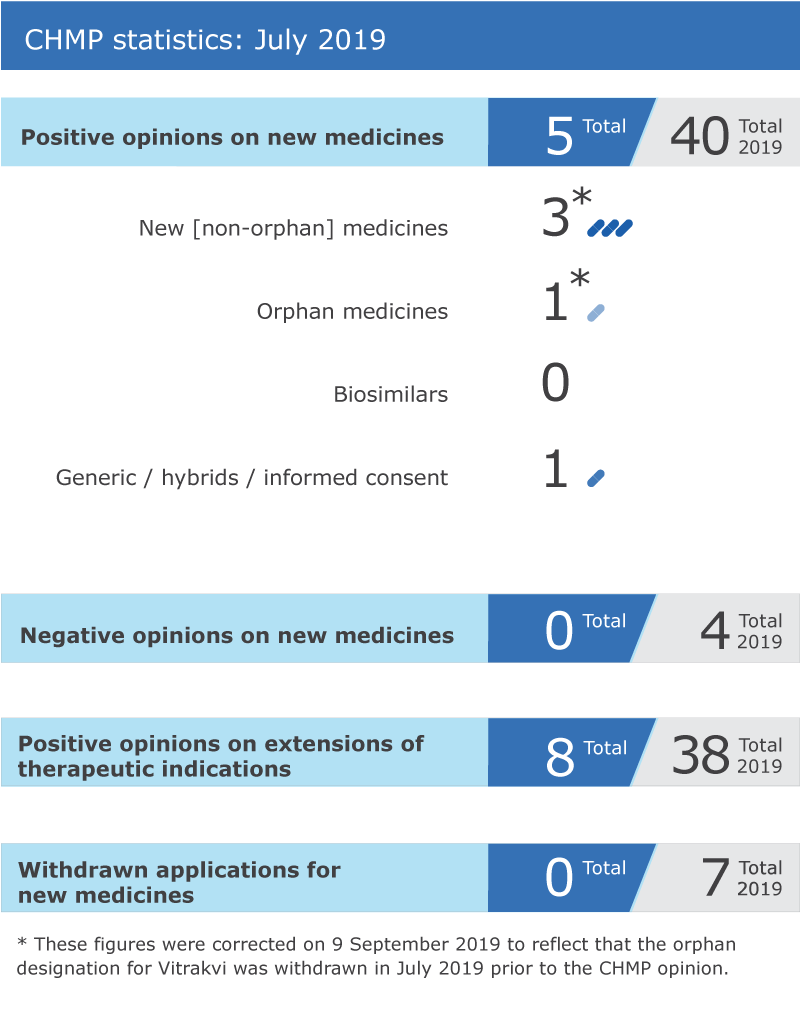
CHMP statistics
Key figures from the July 2019 CHMP meeting are represented in the graphic below.
*This product was designated as an orphan medicine during its development. Orphan designations are reviewed by EMA's Committee for Orphan Medicinal Products (COMP) at the time of approval to determine whether the information available to date allows maintaining the medicine's orphan status and granting the medicine ten years of market exclusivity.
Positive recommendations on new medicines
| Name of medicine | Epidyolex |
| INN | cannabidiol |
| Marketing-authorisation applicant | GW Pharma (International) B.V. |
| Therapeutic indication | Treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome |
| More information | Epidyolex: Pending EC decision |
| Name of medicine | Inbrija |
| INN | levodopa |
| Marketing-authorisation applicant | Acorda Therapeutics Ireland Limited |
| Therapeutic indication | Treatment of symptoms of off periods in Parkinson’s disease |
| More information | Inbrija: Pending EC decision |
| Name of medicine | Trogarzo |
| INN | ibalizumab |
| Marketing-authorisation applicant | Theratechnologies International Limited |
| Therapeutic indication | Treatment of HIV infection |
| More information | Trogarzo: Pending EC decision |
| Name of medicine | Vitrakvi |
| INN | larotrectinib |
| Marketing-authorisation applicant | Bayer AG |
| Therapeutic indication | Treatment of patients with solid tumours that display a neurotrophic tyrosine receptor kinase (NTRK) gene fusion |
| More information | Vitrakvi: Pending EC decision |
| Press release | First ‘histology-independent’ treatment for solid tumours with a specific gene mutation |
Positive recommendation on new generic medicine
| Name of medicine | Deferasirox Mylan |
| International non-proprietary name (INN) | deferasirox |
| Marketing-authorisation applicant | Mylan S.A.S. |
| Therapeutic indication | Treatment of chronic iron overload due to blood transfusions in patients with beta thalassaemia major, non-transfusion-dependent thalassaemia syndromes and other anaemias |
| More information | Deferasirox Mylan: Pending EC decision |
Start of re-examination of recommendation for new medicine
| Name of medicine | Evenity |
| INN | romosozumab |
| Marketing-authorisation applicant | UCB Pharma S.A. |
| Therapeutic indication | Treatment of osteoporosis |
| More information | Evenity: Pending EC decision |
Positive recommendations on extensions of indications
| Name of medicine | Empliciti |
| INN | elotuzumab |
| Marketing-authorisation holder | Bristol-Myers Squibb Pharma EEIG |
| More information | Empliciti: Pending EC decision |
| Name of medicine | Keytruda |
| INN | pembrolizumab |
| Marketing-authorisation holder | Merck Sharp & Dohme B.V. |
| More information | Keytruda: Pending EC decision |
| Name of medicine | Lonsurf |
| INN | trifluridine / tipiracil |
| Marketing-authorisation holder | Les Laboratoires Servier |
| More information | Lonsurf: Pending EC decision |
| Name of medicine | Lucentis |
| INN | ranibizumab |
| Marketing-authorisation holder | Novartis Europharm Limited |
| More information | Lucentis: Pending EC decision |
| Name of medicine | Soliris |
| INN | eculizumab |
| Marketing-authorisation holder | Alexion Europe SAS |
| More information | Soliris: Pending EC decision |
| Name of medicine | Stelara |
| INN | ustekinumab |
| Marketing-authorisation holder | Janssen-Cilag International NV |
| More information | Stelara: Pending EC decision |
| Name of medicine | Tecentriq |
| INN | atezolizumab |
| Marketing-authorisation holder | Roche Registration GmbH |
| More information | Tecentriq: Pending EC decision |
| Name of medicine | Zerbaxa |
| INN | ceftolozane / tazobactam |
| Marketing-authorisation holder | Merck Sharp & Dohme B.V. |
| More information | Zerbaxa: Pending EC decision |
Start of re-examination of extension of indication
| Name of medicine | Revolade |
| INN | eltrombopag |
| Marketing-authorisation holder | Novartis Europharm Limited |
| More information | Revolade: Pending EC decision |
| Name of medicine | Translarna |
| INN | ataluren |
| Marketing-authorisation holder | PTC Therapeutics International Limited |
| More information | Translarna: Pending EC decision |
Public-health recommendation
| Name of medicine | Gilenya |
| INN | fingolimod |
| More information | Updated restrictions for Gilenya: multiple sclerosis medicine not to be used in pregnancy |