Three medicines recommended for approval
The European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) recommended three medicines for approval at its April 2018 meeting.
The CHMP recommended granting a marketing authorisation for Biktarvy (bictegravir / emtricitabine / tenofovir alafenamide), for the treatment of HIV-1 infection.
One hybrid medicine, Dzuveo (sufentanil) received a positive opinion for the treatment of pain. Hybrid applications rely in part on the results of pre-clinical tests and clinical trials for a reference product and in part on new data.
The Committee recommended for approval the generic medicine Carmustine Obvius (carmustine), for the treatment brain tumours, non-Hodgkin's lymphoma and Hodgkin's disease.
Start of re-examination of recommendations on new medicines
The applicants for Dexxience (betrixaban) and Eladynos (abaloparatide) have requested re-examinations of the CHMP's negative opinions for these medicines adopted at the March 2018 meeting.
The applicant for Alsitek (masitinib) has also requested a re-examination of the Committee's negative opinion for this medicine, which was adopted via written procedure on 18 April 2018.
The CHMP will now re-examine these opinions and issue final recommendations. For more information on the negative opinions, please see the question-and-answer documents in the grid below.
Eight recommendations on extensions of therapeutic indication
The Committee recommended extensions of indications for Cimzia, Perjeta, Prolia, Sprycel, Tagrisso, Xeljanz, Xultophy and Yervoy.
Withdrawals of applications
The application for an initial marketing authorisation for Prohippur (sodium benzoate) was withdrawn. This medicine was intended to be used for the treatment of non-ketotic hyperglycinaemia and urea cycle disorders.
An application to extend the use of Qtern (saxagliptin / dapagliflozin) in patients with type 2 diabetes has also been withdrawn.
Question-and-answer documents on these withdrawals are available in the grid below.
Agenda and minutes
The agenda of the April 2018 meeting is published on EMA's website. Minutes of the March 2018 CHMP meeting will be published in the coming weeks.
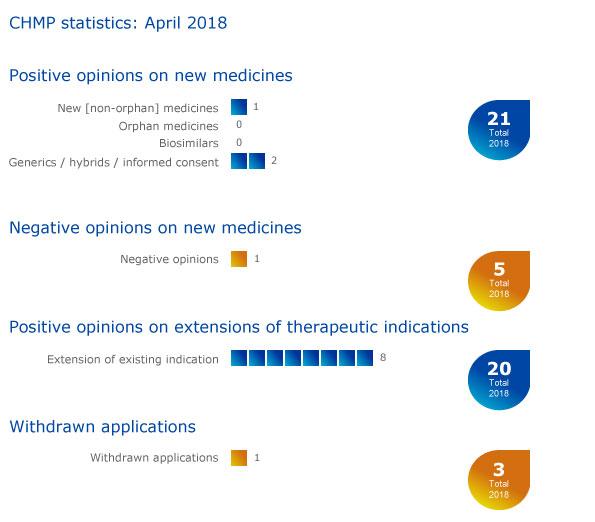
CHMP statistics
Key figures from the April 2018 CHMP meeting are represented in the graphic below.
More information on all other outcomes of the CHMP April 2018 meeting is available in the grid below.
Download image in PDF format
Positive recommendations on new medicines
| Name of medicine | Biktarvy |
|---|---|
| International non-proprietary name (INN) | bictegravir / emtricitabine / tenofovir alafenamide |
| Marketing-authorisation applicant | Gilead Sciences International Limited |
| Therapeutic indication | Treatment of HIV-1 infection |
| More information | CHMP summary of positive opinion for Biktarvy |
| Name of medicine | Dzuveo |
|---|---|
| INN | sufentanil |
| Marketing-authorisation applicant | FGK Representative Service GmbH |
| Therapeutic indication | Treatment of pain |
| More information | CHMP summary of positive opinion for Dzuveo |
Positive recommendation on new generic medicine
| Name of medicine | Carmustine Obvius |
|---|---|
| INN | carmustine |
| Marketing-authorisation applicant | Obvius Investment BV |
| Therapeutic indication | Treatment of brain tumours, non-Hodgkin's lymphoma and Hodgkin's disease |
| More information | CHMP summary of positive opinion for Carmustine Obvius |
Start of re-examination of recommendation for new medicines
| Name of medicine | Alsitek* |
|---|---|
| INN | masitinib |
| Marketing-authorisation applicant | AB Science |
| Therapeutic indication | Treatment of amyotrophic lateral sclerosis |
| More information | Questions and answers on refusal of the marketing authorisation for Alsitek (masitinib)
* The CHMP adopted a negative opinion for this medicine via written procedure on 18 April 2018 |
| Name of medicine | Dexxience |
|---|---|
| INN | betrixaban |
| Marketing-authorisation applicant | Portola Pharma UK Limited |
| Therapeutic indication | Prevention of venous thromboembolism |
| More information | Questions and answers on refusal of the marketing authorisation for Dexxience (betrixaban) |
| Name of medicine | Eladynos |
|---|---|
| INN | abaloparatide |
| Marketing-authorisation applicant | Radius International Ltd |
| Therapeutic indication | Treatment of osteoporosis |
| More information | Questions and answers on refusal of the marketing authorisation for Eladynos (abaloparatide) |
Positive recommendations on extensions of indications
| Name of medicine | Cimzia |
|---|---|
| INN | certolizumab pegol |
| Marketing-authorisation holder | UCB Pharma SA |
| More information | CHMP post-authorisation summary of positive opinion for Cimzia (II-65) |
| Name of medicine | Perjeta |
|---|---|
| INN | pertuzumab |
| Marketing-authorisation holder | Roche Registration GmbH |
| More information | CHMP post-authorisation summary of positive opinion for Perjeta (II-0034) |
| Name of medicine | Prolia |
|---|---|
| INN | denosumab |
| Marketing-authorisation holder | Amgen Europe B.V. |
| More information | CHMP post-authorisation summary of positive opinion for Prolia (II-68) |
| Name of medicine | Sprycel |
|---|---|
| INN | dasatinib |
| Marketing-authorisation holder | Bristol-Myers Squibb Pharma EEIG |
| More information | Sprycel |
| Name of medicine | Tagrisso |
|---|---|
| INN | osimertinib |
| Marketing-authorisation holder | AstraZeneca AB |
| More information | CHMP post-authorisation summary of positive opinion for Tagrisso II/0019 |
| Name of medicine | Xeljanz |
|---|---|
| INN | tofacitinib |
| Marketing-authorisation holder | Pfizer Limited |
| More information | CHMP post-authorisation summary of positive opinion for Xeljanz |
| Name of medicine | Xultophy |
|---|---|
| INN | insulin degludec / liraglutide |
| Marketing-authorisation holder | Novo Nordisk A/S |
| More information | CHMP post-authorisation summary of positive opinion for Xultophy II/23 |
| Name of medicine | Yervoy |
|---|---|
| INN | ipilimumab |
| Marketing-authorisation holder | Bristol-Myers Squibb Pharma EEIG |
| More information | CHMP post-authorisation summary of positive opinion for Yervoy (II-55) |
Withdrawal of initial marketing authorisation application
| Name of medicine | Prohippur |
|---|---|
| INN | sodium benzoate |
| Marketing-authorisation applicant | Lucane Pharma |
| More information | Questions and answers on the withdrawal of the marketing authorisation for Prohippur (sodium benzoate) |
Withdrawal of extension of indication application
| Name of medicine | Qtern |
|---|---|
| INN | saxagliptin / dapagliflozin |
| Marketing-authorisation holder | Astra Zeneca AB |
| More information | Questions and answers on the withdrawal of the application for a change to the marketing authorisation for Qtern (saxagliptin / dapagliflozin) |
Other updates