EMA’s human medicines committee (CHMP) recommended eight medicines for approval, including two orphan medicines1, at its February 2019 meeting. For three of the eight new medicines the CHMP recommended a conditional marketing authorisation.
This was the last meeting of the CHMP in London. As of March 2019, all meetings will take place at the new EMA headquarters in Amsterdam.
The Committee recommended granting a conditional marketing authorisation for Ondexxya (andexanet alfa). This medicine is to be used as an antidote for adult patients taking the anticoagulant medicines apixaban or rivaroxaban, when reversal of their action is needed due to life-threatening or uncontrolled bleeding. For more information, please see the press release in the grid below.
The Committee adopted a positive opinion for Palynziq (pegvaliase), a new medicine for patients aged 16 and older with phenylketonuria, a rare but potentially serious inherited metabolic disease. Palynziq was designated as an orphan medicine during its development. For more information, please see the press release in the grid below.
The CHMP recommended granting a conditional marketing authorisation for Waylivra (volanesorsen), the first medicine for the treatment of familial chylomicronaemia syndrome, a rare genetic disease that prevents the body from breaking down fats. Waylivra was designated as an orphan medicine during its development. For more information, please see the press release in the grid below.
Zynquista (sotagliflozin), intended as an adjunct to insulin for certain patients with type 1 diabetes, received a positive opinion from the CHMP. For more information, please see the press release in the grid below.
The Committee recommended granting a marketing authorisation under exceptional circumstances for Dectova (zanamivir), for the treatment of complicated and potentially life-threatening influenza.
The CHMP adopted a positive opinion, recommending the granting of a conditional marketing authorisation for Lorviqua (lorlatinib), for the treatment of patients with anaplastic lymphoma kinase-positive advanced non-small cell lung cancer.
Skyrizi (risankizumab) received a positive opinion for the treatment of moderate to severe psoriasis.
The Committee recommended for approval the generic medicine Pazenir (paclitaxel), for the treatment of metastatic breast cancer and non-small cell lung cancer.
Start of re-examination of recommendation for new medicine
The applicant for Doxolipad (doxorubicin) has requested a re-examination of the Committee's negative opinion for this medicine adopted at the January 2019 meeting. The CHMP will now re-examine the opinion and issue a final recommendation.
For more information on this negative opinion, please see the question-and-answer document in the grid below.
Five recommendations on extensions of therapeutic indication
The CHMP recommended an extension of indication of Dupixent as an add-on maintenance treatment for patients 12 years and older with certain forms of severe asthma. For more information, please see the press release in the grid below.
Other extensions of indication recommended by the Committee were for Lynparza, Riarify, Trydonis and Viread.
Withdrawals of application
The application for an initial marketing authorisation for Epjevy (pacritinib citrate) was withdrawn. This medicine was intended to be used to treat symptoms of myelofibrosis in patients with severe thrombocytopenia.
A question-and-answer document on this withdrawal is available in the grid below.
Agenda and minutes
The agenda of the February 2019 meeting is published on EMA's website. Minutes of the January 2019 CHMP meeting will be published in the coming weeks.
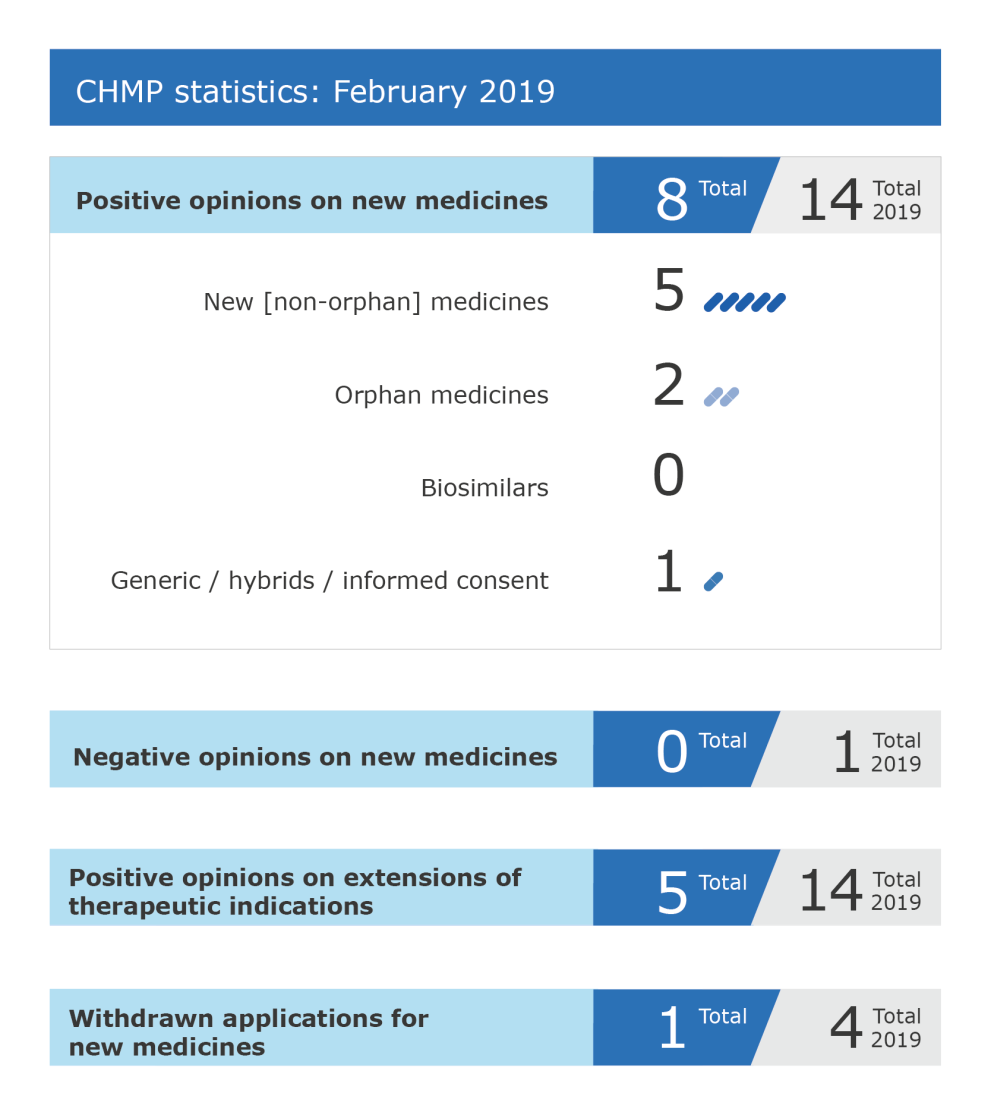
CHMP statistics
Key figures from the February 2019 CHMP meeting are represented in the graphic below.
1As always at time of approval, these orphan designations will now be reviewed by EMA's Committee for Orphan Medicinal Products (COMP) to determine whether the information available to date allows maintaining the medicines' orphan status and granting the medicines ten years of market exclusivity.
Positive recommendations on new medicines
| Name of medicine | Dectova |
|---|---|
| International non-proprietary name (INN) | zanamivir |
| Marketing-authorisation applicant | GlaxoSmithKline Trading Services Limited |
| Therapeutic indication | Treatment of complicated and potentially life-threatening influenza. |
| Name of medicine | Lorviqua |
|---|---|
| INN | lorlatinib |
| Marketing-authorisation applicant | Pfizer Europe MA EEIG |
| Therapeutic indication | Treatment of patients with anaplastic lymphoma kinase (ALK)-positive advanced non-small cell lung cancer (NSCLC). |
| Name of medicine | Ondexxya |
|---|---|
| INN | andexanet alfa |
| Marketing-authorisation applicant | Portola Netherlands B.V. |
| Therapeutic indication | Treatment of direct factor Xa(FXa) inhibitor (apixaban or rivaroxaban) when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding. |
| More information | First antidote for reversal of anticoagulation with factor Xa inhibitors apixaban and rivaroxaban |
| Name of medicine | Palynziq |
|---|---|
| INN | pegvaliase |
| Marketing-authorisation applicant | BioMarin International Limited |
| Therapeutic indication | Treatment of phenylketonuria. |
| More information | CHMP recommends authorisation of new treatment for phenylketonuria, a rare inherited metabolic disease |
| Name of medicine | Skyrizi |
|---|---|
| INN | risankizumab |
| Marketing-authorisation applicant | AbbVie Deutschland GmbH & Co. KG |
| Therapeutic indication | Treatment of moderate to severe psoriasis. |
| Name of medicine | Waylivra |
|---|---|
| INN | volanesorsen |
| Marketing-authorisation applicant | Akcea Therapeutics Ireland Ltd |
| Therapeutic indication | Treatment of familial chylomicronaemia syndrome. |
| More information | First treatment for rare disease characterised by high levels of triglycerides in blood |
| Name of medicine | Zynquista |
|---|---|
| INN | sotagliflozin |
| Marketing-authorisation applicant | sanofi-aventis groupe |
| Therapeutic indication | Treatment of type 1 diabetes mellitus as an adjunct to insulin. |
| More information | New add-on treatment to insulin for treatment of certain patients with type 1 diabetes |
Positive recommendation on new generic medicine
| Name of medicine | Pazenir (previously known as Paclitaxel Teva Pharma) |
|---|---|
| INN | paclitaxel |
| Marketing-authorisation applicant | Teva B.V. |
| Therapeutic indication | Treatment of metastatic breast cancer and non-small cell lung cancer. |
Start of re-examination of recommendation for new medicine
| Name of medicine | Doxolipad |
|---|---|
| INN | doxorubicin |
| Marketing-authorisation applicant | TLC Biopharmaceuticals B.V. |
| Therapeutic indication | Treatment of breast and ovarian cancer. |
Positive recommendations on extensions of indications
| Name of medicine | Dupixent |
|---|---|
| INN | dupilumab |
| Marketing-authorisation holder | sanofi-aventis groupe |
| More information | New add-on treatment for patients with severe asthma |
| Name of medicine | Lynparza |
|---|---|
| INN | olaparib |
| Marketing-authorisation holder | AstraZeneca AB |
| Name of medicine | Riarify |
|---|---|
| INN | beclometasone dipropionate / formoterol fumarate dihydrate / glycopyrronium |
| Marketing-authorisation holder | Chiesi Farmaceutici S.p.A. |
| Name of medicine | Trydonis |
|---|---|
| INN | beclometasone dipropionate / formoterol fumarate dihydrate / glycopyrronium |
| Marketing-authorisation holder | Chiesi Farmaceutici S.p.A. |
| Name of medicine | Viread |
|---|---|
| INN | tenofovir disoproxil |
| Marketing-authorisation holder | Gilead Sciences Ireland UC |
Outcome of arbitration procedure
| Name of medicine | Syner-KINASE 10 000 IU |
|---|---|
| More information | Syner-Kinase and associated names |
| Name of medicine | Epjevy |
|---|---|
| INN | pacritinib citrate |
| Marketing-authorisation applicant | CTI Life Sciences Limited |
| More information | Epjevy: Withdrawn application |