One of the European Medicines Agency's (EMA) strategic goals is to promote development of innovative veterinary medicines and new technologies. Technologies that are new to veterinary medicine present particular challenges due to a lack of regulatory guidance and, in some cases, the fact that the existing regulatory framework does not specifically cater for them.
EMA's Committee for Veterinary Medicinal Products (CVMP) has established the Novel Therapies and Technologies Working Party to provide guidance on the requirements for authorisation of novel veterinary therapies.
The image below gives an overview of the various tools EMA has set up to provide scientific and regulatory support to those developing innovative veterinary medicines.
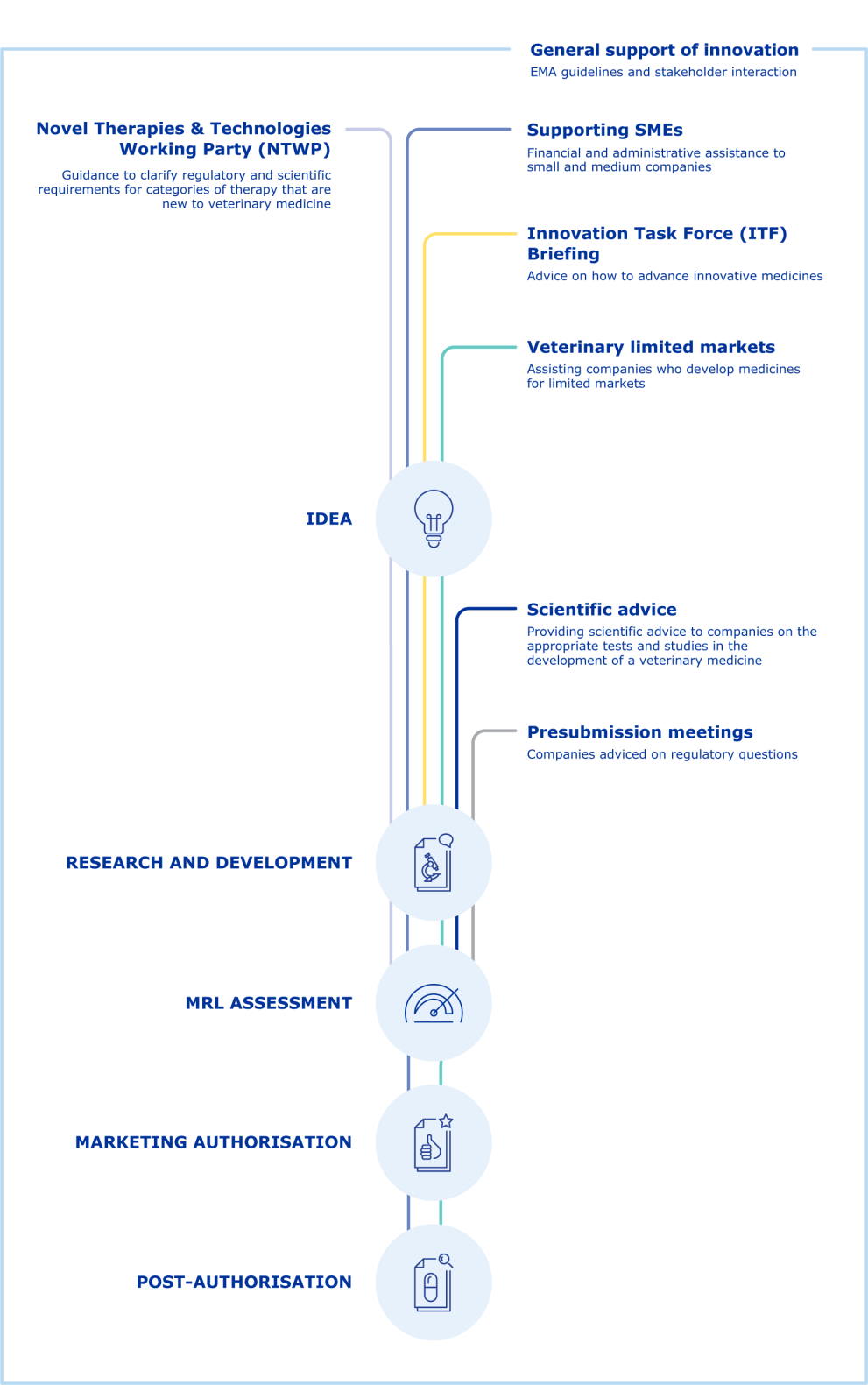
EMA support for innovative veterinary medicines (includes links to relevant pages)
For further information on innovation in medicines, including information on the Innovation Task Force (ITF) and how to request an ITF briefing meeting, please refer to the content in the human regulatory section:
See also: