Seven new medicines recommended for approval
EMA’s human medicines committee (CHMP) recommended seven medicines for approval at its April 2023 meeting.
The CHMP recommended granting a marketing authorisation for Arexvy (recombinant, adjuvanted), the first vaccine for active immunisation to protect adults aged 60 years and older against lower respiratory tract disease caused by respiratory syncytial virus (RSV). RSV is a common respiratory virus that usually causes mild, cold-like symptoms that can be serious in vulnerable people, including older adults and those with lung or heart disease and diabetes. See more information in the news announcement in the grid below.
The Committee gave a positive opinion for Camzyos (mavacamten) for the treatment of symptomatic obstructive hypertrophic cardiomyopathy, a disease in which the heart muscle becomes thickened and can make it harder for the heart to pump blood.
A positive opinion was adopted for Columvi* (glofitamab) under conditional marketing authorisation for the treatment of diffuse large B-cell lymphoma, an aggressive type of non-Hodgkin lymphoma, a cancer of the lymphatic system that can arise in lymph nodes or outside of the lymphatic system.
Jaypirca* (pirtobrutinib) received a positive opinion under conditional marketing authorisation from the CHMP for the treatment of relapsed or refractory mantle cell lymphoma which develops when B-cells, a type of white blood cell that makes antibodies, become abnormal.
The CHMP gave a positive opinion for Lytgobi* (futibatinib), recommending the granting of a conditional marketing authorisation1, for the treatment of cholangiocarcinoma or bile duct cancer, a type of cancer that forms in the slender tubes that carry the digestive fluid.
The Committee recommended Opfolda (miglustat) for the treatment of glycogen storage disease type II (Pompe disease) in combination with cipaglucosidase alfa. Pompe disease is a rare genetic disorder in which the body is not able to break down glycogen, leading to progressive build-up that causes a wide range of symptoms, including an enlarged heart, breathing difficulties and muscle weakness. This medicine was submitted as a hybrid application, which relies in part on pre-clinical and clinical data of an already authorised reference product and in part on results of new studies.
A positive opinion was adopted for Sugammadex Piramal (sugammadex), a generic medicine indicated for the treatment of the reversal of neuromuscular blockade induced by rocuronium or vecuronium in adults. Rocuronium and vecuronium are muscle relaxants used during some types of surgeries requiring general anaesthesia. Sugammadex is used to speed up the recovery from the effects of the muscle relaxant.
Recommendations on extensions of therapeutic indication for ten medicines
The Committee recommended 11 extensions of indication for medicines that are already authorised in the European Union (EU): Adempas, Bimzelx (includes two new indications), Cosentyx, Opdivo, Orkambi, Revestive*, Ronapreve, Spikevax, Vemlidy, Yervoy.
More information on the extensions of indication for the COVID-19 treatment Ronapreve and the COVID-19 vaccine Spikevax is available below.
Withdrawals of applications
The application for marketing authorisation for the advanced therapy medicinal product Lumevoq* was withdrawn. The medicine was intended for the treatment of vision loss due to Leber hereditary optic neuropathy. A question-and-answer documents on the withdrawal for Lumevoq is available in the grid below.
The application for marketing authorisation for Tidhesco* was withdrawn. This medicine was intended for the treatment of acute myeloid leukaemia. Tidhesco was a duplicate of Tibsovo. Both applications received a positive opinion on 23 February 2023.
Other updates
Concluding the assessment of an application to extend the use of Epidyolex* (cannabidiol), the CHMP recommended that the medicine should continue to be administered only in conjunction with clobazam for adjunctive therapy of seizures associated with Lennox-Gastaut syndrome (LGS) or Dravet syndrome (DS) in patients two years of age and older. LSG and DS are severe forms of childhood epilepsy. A question-and-answer document on the update is available in the grid below.
The CHMP endorsed an update to the Statement on the Interchangeability of Biosimilar medicines in the EU to stress that healthcare professionals and patients should carefully consider the product information before the decision to interchange a biosimilar treatment. The updated statement and an expanded question-and-answer document including these revisions are available in the grid below.
COVID-19 updates
The Committee recommended granting an extension of indication for Spikevax bivalent Original/Omicron BA.4-5 to include the use of this COVID-19 vaccine as a booster in children aged six to 11.
The CHMP also recommended an extension of indication for Ronapreve to include treatment of COVID-19 in hospitalised adults and adolescents aged 12 years and older weighing at least 40 kg who are receiving supplemental oxygen and have a negative SARS-CoV-2 antibody test.
Agenda and minutes
The Agenda of the CHMP meeting 24-26 April 2023 is published on EMA's website. Minutes of the March 2023 CHMP meeting will be published in the coming weeks.
CHMP statistics
Key figures from the April 2023 CHMP meeting are represented in the graphic below.
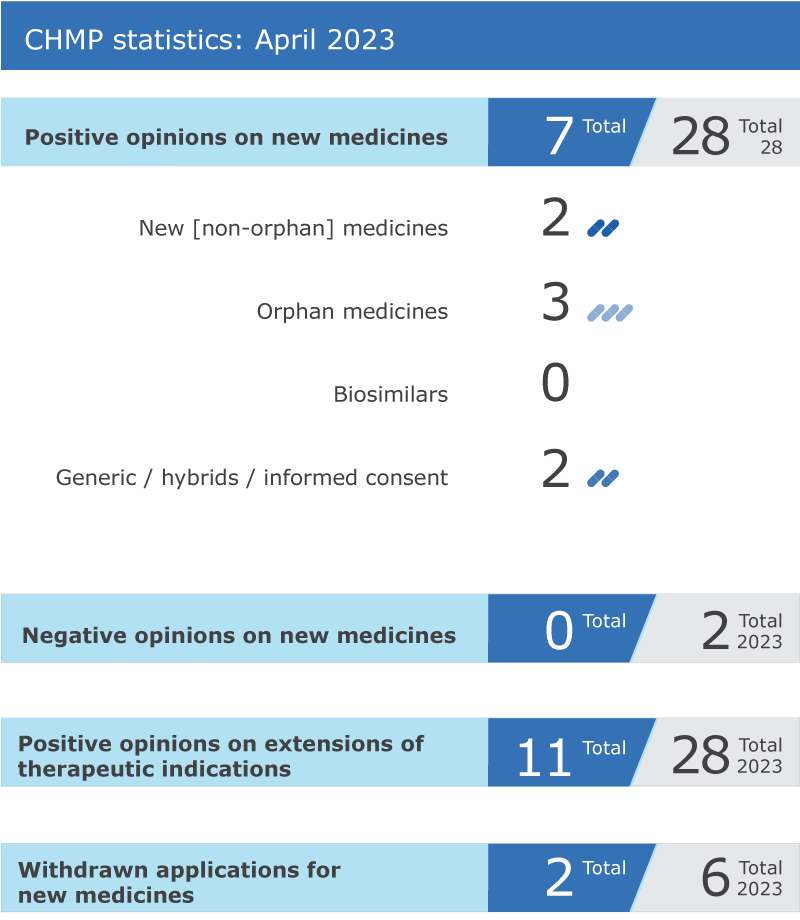
*This product was designated as an orphan medicine during its development. Orphan designations are reviewed by EMA's Committee for Orphan Medicinal Products (COMP) at the time of approval to determine whether the information available to date allows maintaining the medicine’s orphan status and granting the medicine ten years of market exclusivity.
1The information for Lytgobi was corrected on26 May 2023 to reflect that the product has received a positive opinion for a conditional marketing authorisation (and not a full marketing authorisation as stated on 26 April 2023).
Positive recommendations on new medicines
| Name of medicine | Arexvy |
|---|---|
| Common name | Recombinant, adjuvanted |
| Marketing-authorisation applicant | GlaxoSmithkline Biologicals S.A. |
| Therapeutic indication | Active immunisation for the prevention of lower respiratory tract disease (LRTD) caused by RSV (new active substance) |
| More information | News: First vaccine to protect older adults from respiratory syncytial virus (RSV) infection (26/04/2023) |
| Name of medicine | Camzyos |
|---|---|
| INN | mavacamten |
| Marketing-authorisation applicant | Bristol-Myers Squibb Pharma EEIG |
| Therapeutic indication | Treatment of symptomatic obstructive hypertrophic cardiomyopathy |
| More information | Camzyos: Pending EC decision |
| Name of medicine | Columvi |
|---|---|
| INN | glofitamab |
| Marketing-authorisation applicant | Roche Registration GmbH |
| Therapeutic indication | Treatment of diffuse large B-cell lymphoma (new active substance) |
| More information | Columvi: Pending EC decision |
| Name of medicine | Jaypirca |
|---|---|
| INN | pirtobrutinib |
| Marketing-authorisation applicant | Eli Lilly Nederland B.V. |
| Therapeutic indication | Treatment of mantle cell lymphoma (MCL) (new active substance) |
| More information | Jaypirca: Pending EC decision |
| Name of medicine | Lytgobi |
|---|---|
| INN | futibatinib |
| Marketing-authorisation applicant | Taiho Pharma Netherlands B.V. |
| Therapeutic indication | Treatment of cholangiocarcinoma (new active substance) |
| More information | Lytgobi: Pending EC decision |
Positive recommendation on new hybrid medicine
| Name of medicine | Opfolda |
|---|---|
| INN | miglustat |
| Marketing-authorisation applicant | Amicus Therapeutics Europe Limited |
| Therapeutic indication | Treatment of glycogen storage disease type II (Pompe disease) in combination with cipaglucosidase alfa |
| More information | Opfolda: Pending EC decision |
Positive recommendation on new generic medicine
| Name of medicine | Sugammadex Piramal |
|---|---|
| INN | sugammadex |
| Marketing-authorisation applicant | Piramal Critical Care B.V. |
| Therapeutic indication | Reversal of neuromuscular blockade induced by rocuronium or vecuronium in adults (generic of Bridion) |
| More information | Sugammadex Piramal: Pending EC decision |
Positive recommendations on extensions of indications
| Name of medicine | Adempas |
|---|---|
| INN | riociguat |
| Marketing-authorisation holder | Bayer AG |
| More information | Adempas: Pending EC decision |
| Name of medicine | Bimzelx |
|---|---|
| INN | bimekizumab |
| Marketing-authorisation holder | UCB Pharma S.A. |
| More information | Bimzelx: Pending EC decision (II/0010) |
| Name of medicine | Bimzelx |
|---|---|
| INN | bimekizumab |
| Marketing-authorisation holder | UCB Pharma S.A. |
| More information | Bimzelx: Pending EC decision (II/0011) |
| Name of medicine | Cosentyx |
|---|---|
| INN | secukinumab |
| Marketing-authorisation holder | Novartis Europharm Limited |
| More information | Cosentyx: Pending EC decision |
| Name of medicine | Opdivo |
|---|---|
| INN | nivolumab |
| Marketing-authorisation holder | Bristol-Myers Squibb Pharma EEIG |
| More information | Opdivo: Pending EC decision |
| Name of medicine | Orkambi |
|---|---|
| INN | lumacaftor / ivacaftor |
| Marketing-authorisation holder | Vertex Pharmaceuticals (Ireland) Limited |
| More information | Orkambi: Pending EC decision |
| Name of medicine | Revestive |
|---|---|
| INN | teduglutide |
| Marketing-authorisation holder | Takeda Pharmaceuticals International AG Ireland Branch |
| More information | Revestive: Pending EC decision |
| Name of medicine | Ronapreve |
|---|---|
| INN | casirivimab / imdevimab |
| Marketing-authorisation holder | Roche Registration GmbH |
| More information | Ronapreve: Pending EC decision |
| Name of medicine | Spikevax |
|---|---|
| INN | elasomeran |
| Marketing-authorisation holder | Moderna Biotech Spain, S.L. |
| More information | Spikevax: Pending EC decision |
| Name of medicine | Vemlidy |
|---|---|
| INN | tenofovir alafenamide |
| Marketing-authorisation holder | Gilead Sciences Ireland UC |
| More information | Vemlidy: Pending EC decision |
| Name of medicine | Yervoy |
|---|---|
| INN | ipilimumab |
| Marketing-authorisation holder | Bristol-Myers Squibb Pharma EEIG |
| More information | Yervoy: Pending EC decision |
| Name of medicine | Lumevoq |
|---|---|
| INN | lenadogene nolparvovec |
| Marketing-authorisation applicant | GenSight Biologics S.A. |
| More information | Lumevoq: Withdrawn application |
| Name of medicine | Tidhesco |
|---|---|
| INN | ivosidenib |
| Marketing-authorisation applicant | Les Laboratoires Servier |
| More information | This application was a duplicate of Tibsovo, for which a positive opinion was adopted on 23 February 2023 |