Healthcare professionals
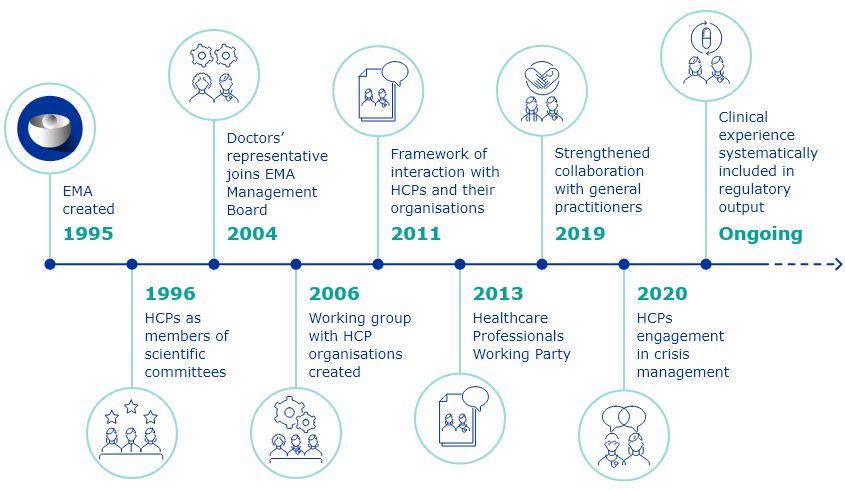
The European Medicines Agency (EMA) has been interacting with European healthcare professionals in various areas of its work since it was founded in 1995.
HumanCorporateGovernance
On Monday, 12 January 2026, between 07:00 and 10:00 CET (Amsterdam time), this website will be unavailable due to scheduled maintenance.
Healthcare professionals (HCPs) are key stakeholders in EMA's work.
They prescribe and handle the medicines that EMA evaluates.
They also have specific knowledge and expertise to offer.
EMA is committed to maintaining a strong working relationship with healthcare professionals.
For more information on EMA's work with its partners and networks, see:
December 2021 marked ten years of cooperation between EMA and healthcare professionals under a formal framework for interaction.
A report summarises the experience, including:
EMA is committed to working closely with its expanding network of healthcare professional organisations and encourages all eligible, not-for-profit organisations to get involved.
The framework describes the objectives and the terms of reference for this interaction and aims to:
The framework is in line with EMA's overarching framework for stakeholder relations management.
It was first endorsed by EMA’s Management Board in December 2011 and revised in 2016.
For information on the revisions of this framework, select the expandable panel below:
EMA and the two major organisations representing general practitioners (GPs) together with family physicians in Europe and the major organisation representing primary care professionals in Europe have signed a joint statement committing to strengthening interactions:
The joint statement contains an action plan until 2020.
This includes involving GPs and family physicians in EMA evaluations, developing relevant communication activities and exploring further collaboration with research networks in primary care, with a focus on generating real-world evidence.
It also identifies opportunities for cooperation in regulatory science training.
For more information, see:
Healthcare professionals are involved in a wide range of activities at EMA, including:
For more information, see:
EMA's stakeholder engagement reports provide annual overview of our interactions with patients, consumers, healthcare professionals, academics and their organisations.
These reports incorporate both quantitative and qualitative data.
Read 2022-2023 stakeholder engagement report
To find previous stakeholder engagement reports, see:
EMA's public engagement highlights provide an annual overview of patient and healthcare professional involvement in EMA’s work.
This involvement includes:

To find previous public engagement highlights, see:
EMA is helping ICH to ensure that the perspectives of European patients, healthcare professionals and clinical researchers are taken into account in the ongoing revision of its International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) E6 good clinical practice (GCP) guideline. EMA does so by coordinating the stakeholder engagement process in Europe on the behalf of ICH.
ICH is revising this guideline to make it more responsive to advances in clinical trial design and conduct.
It has committed itself to engaging stakeholders from the outset of the revision process, particularly patient representatives and academic clinical researchers.
This is to ensure that the revised guideline meets the needs of those conducting or participating in clinical trials.
EMA held a workshop with its Patients' and Consumers' (PCWP) and Healthcare Professionals' (HCPWP) working parties in June 2020 to gather their views and experiences. The workshop summary report is available:
For more information, see:

Human Medicines Highlights is an EMA monthly newsletter aimed at patients, consumers and healthcare professionals.
It contains updates on medicines regulation and news for stakeholders.
Issues from May 2024 (Issue 180) onwards are available at the link below:
Previous issues 118-179 are available on EMA's website in PDF format:
Use this link to receive Human Medicines Highlights by email: