
International regulatory cooperation to improve global health
In an increasingly globalised pharmaceutical market and a world in which public health issues go beyond national borders, cooperation among medicine regulators has become key to supervising complex supply chains, avoiding duplication of regulatory work, aligning regulatory approaches and making best use of resources. In 2021, the Agency continued to work with its partners in Europe and beyond to contribute to the health of EU citizens and people around the world.
Bilateral interactions with non-EU regulators
EMA has bilateral confidentiality arrangements with a number of third-country regulators. These arrangements enable the parties to exchange confidential information and provide a framework for regulatory cooperation.
In March 2021, EMA and the European Commission’s Directorate-General for Health and Food Safety (DG SANTE) signed a confidentiality arrangement with the Brazilian Health Regulatory Agency (ANVISA). This brings the number of standing confidentiality agreements to eight.
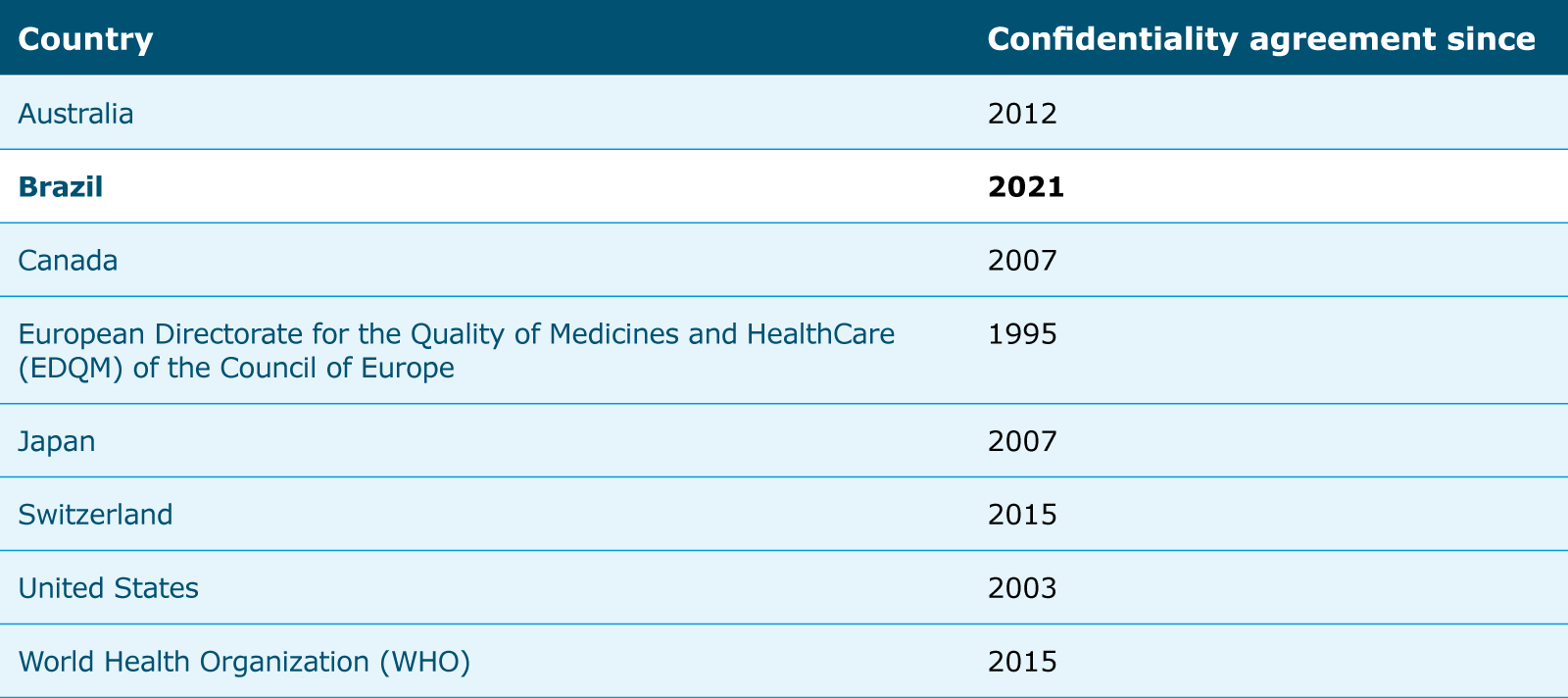
Multilateral work – advancing the role of the International Coalition of Medicines Regulatory Authorities (ICMRA)
Throughout 2021, EMA, as chair of ICMRA, continued to spearhead global efforts to strengthen regulatory cooperation on issues that impact people worldwide. While priority was given to activities related to ensuring alignment of regulatory approaches to the COVID-19 response, ICMRA also covered a range of other activities.
The pandemic has increased the urgency for regulators to converge on responses both to existing regulatory challenges and complex new ones. ICMRA has proven its value during the COVID-19 response, both as a platform for sharing information and best practices and as a venue for providing strategic leadership, active information sharing, pragmatic solutions and regulatory convergence.
EMA led a number of projects and working groups, including supply chain integrity, a horizon-scanning exercise in AI and communications, to achieve ICMRA’s objectives.
In its role as chair of the ICMRA working group on supply chain integrity, EMA contributed to the development of a paper with recommendations to facilitate the use of track and trace systems at global level in 2021. The recommendations were developed in consultation with WHO, representatives from international medicines regulatory authorities and experts from the private sector.
EMA led a horizon-scanning exercise in AI carried out by the ICMRA Informal Network for Innovation working group. The group developed a report with specific recommendations to help regulators address the challenges that the use of AI poses for global medicines regulation.
The Coalition’s visibility increased substantially during 2021, and with it the public awareness of its role in facilitating greater cooperation of international medicines authorities on shared regulatory issues and challenges. This was the result of efforts led by EMA for proactive communications, coordinated campaigns, regular information sharing and cross-channel promotion of ICMRA materials. The Agency published 15 news announcements to inform the public about joint ICMRA initiatives and activities, such as vaccine confidence, transparency and data integrity and regulatory flexibility. As a result, ICMRA was mentioned in 1.4M media articles published globally from January to December 2021.
Increasing ICMRA’s global footprint
Six new members joined ICMRA in 2021, bringing the number up from 31 members in 2020 to 37 participating regulatory authorities representing every region of the world. In addition, WHO participates as an observer.
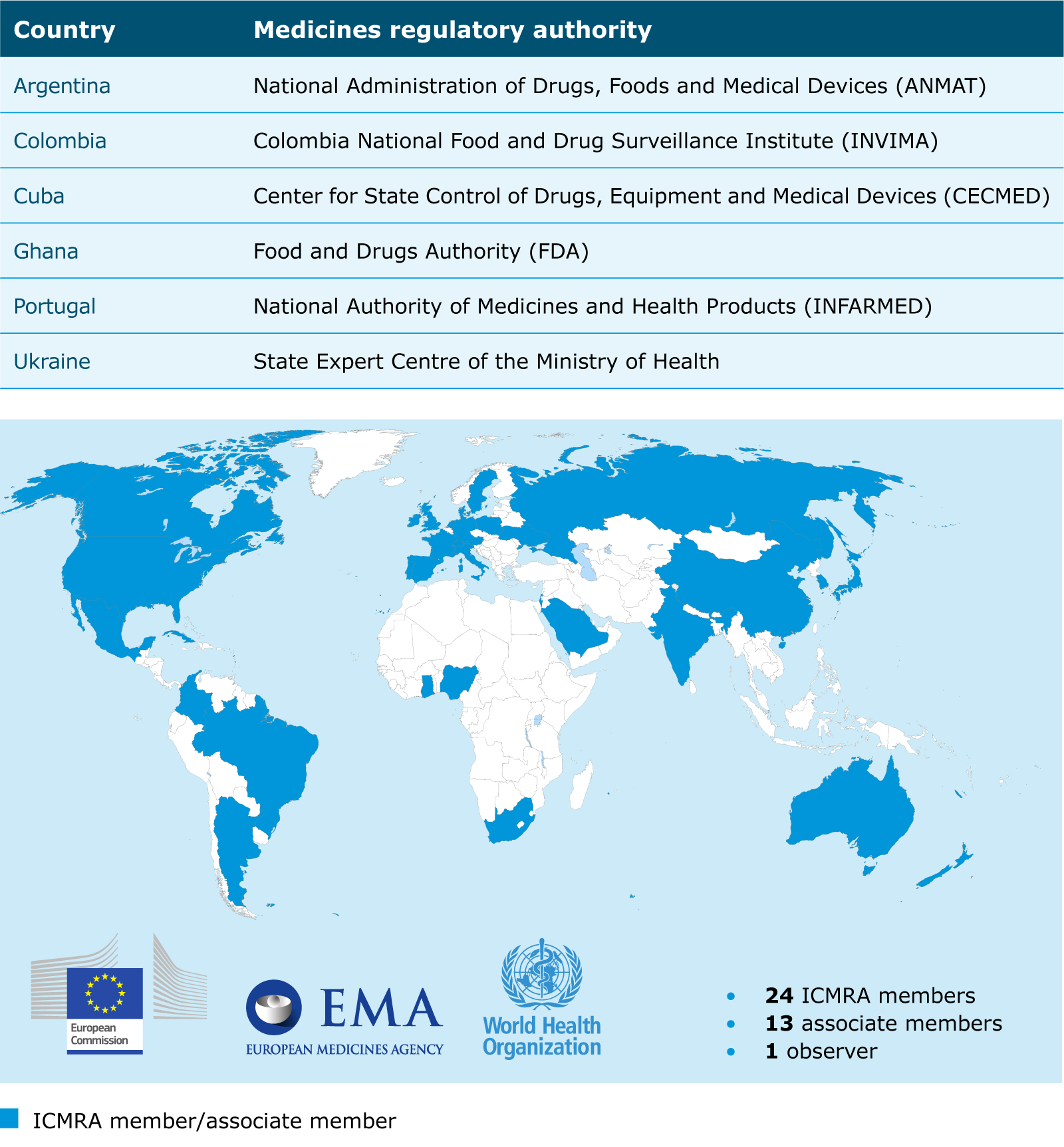
Cooperation with African regulators
EMA is committed to supporting global regulatory capacity building and contributing to the protection and promotion of public health beyond the EU.
In October 2021, EMA shared its assessment of the second Ebola vaccine (which consists of two components, Mvabea and Zabdeno) approved for use in the EU, as part of a review meeting facilitated by WHO. Twenty national regulatory authorities (NRAs) of the most concerned African countries were invited to attend the meeting. The objective of the meeting was to explain in detail EMA’s regulatory assessment of this vaccine and to respond to any questions the invited regulators might have before they take their own regulatory decisions based on EMA’s scientific assessment.
Facilitating access to medicines in low- and middle-income countries
In 2021, EMA analysed the list of approvals granted worldwide based on scientific opinions through the EU-M4all procedure (previously known as Article 58 procedure).
This programme enables EMA, in close cooperation with WHO, to provide scientific opinions on medicines intended for markets outside the EU.
Between 2004 and 2021, EMA issued 12 positive opinions under the EU-M4all procedure, leading to 127 authorisations in 79 countries.
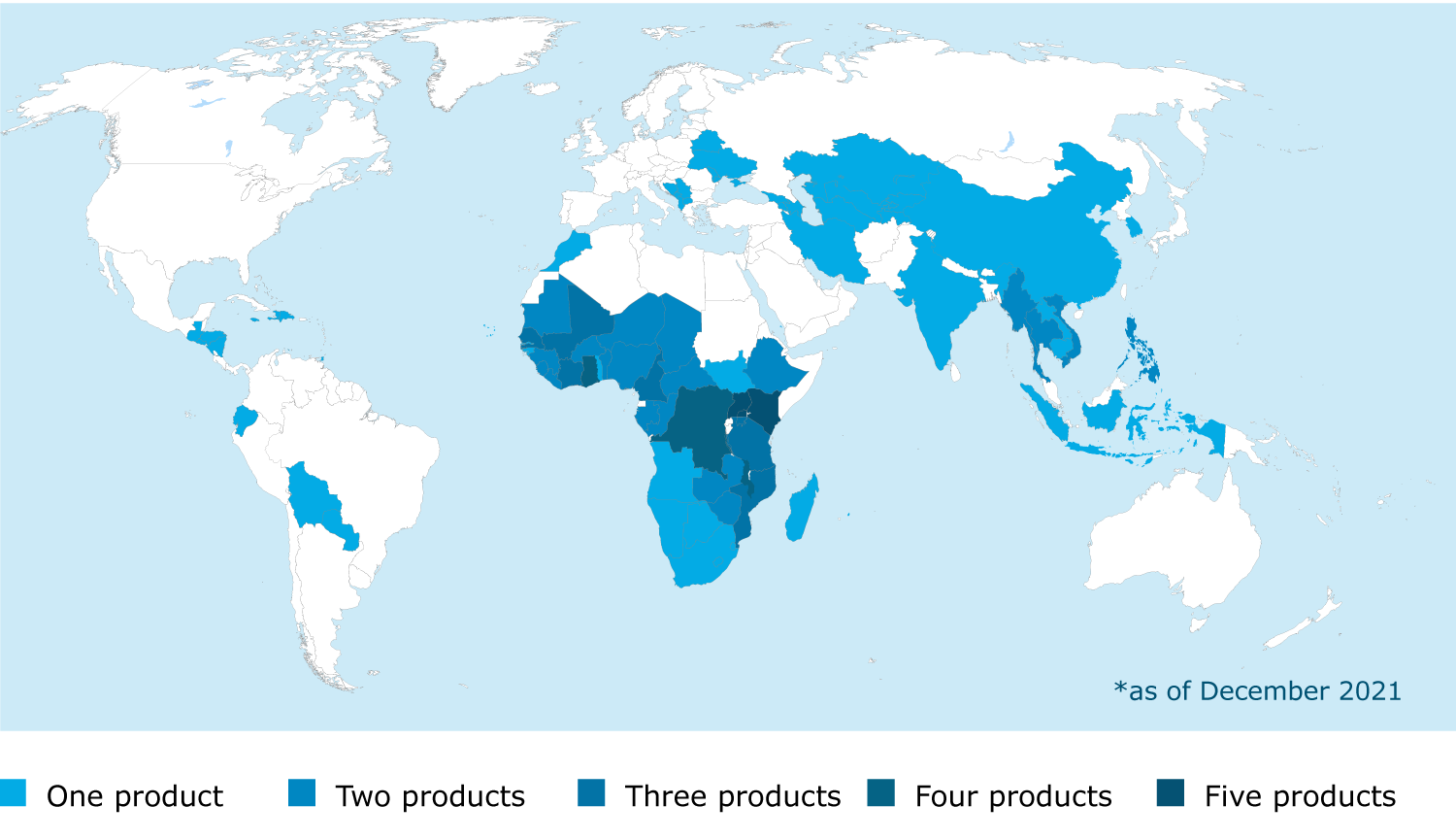
EU-M4all combines EMA’s scientific review capabilities with the epidemiology and disease expertise of WHO and experts from NRAs in the target countries to facilitate the assessment of high-priority medicines, such as new or improved therapies for unmet medical needs, which are intended to prevent or treat diseases of major public health interest.