CHAPTER 2: Key figures in 2024
European medicines regulatory network

The European medicines regulatory network is the cornerstone of EMA’s work and success. EMA plays a central role in this network, coordinating and facilitating collaboration between more than 50 national competent authorities across the EU and EEA for both human and veterinary medicines.
Through the network, EMA can draw from a pool of over 4,000 specialists who provide the highest level of scientific expertise to the regulation of medicines in the EU. These experts contribute to EMA’s scientific committees, working groups and other bodies, and are also involved in the evaluation teams that carry out the evaluation of medicines.
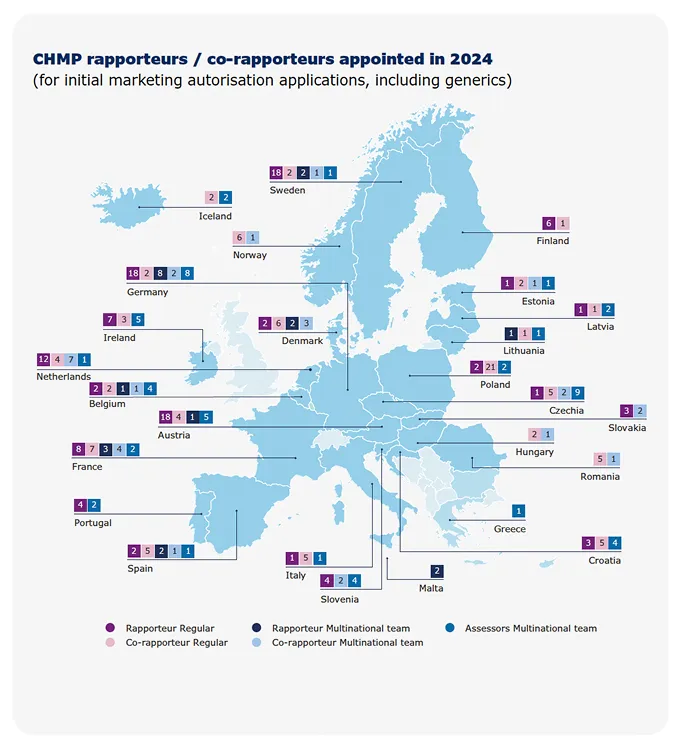
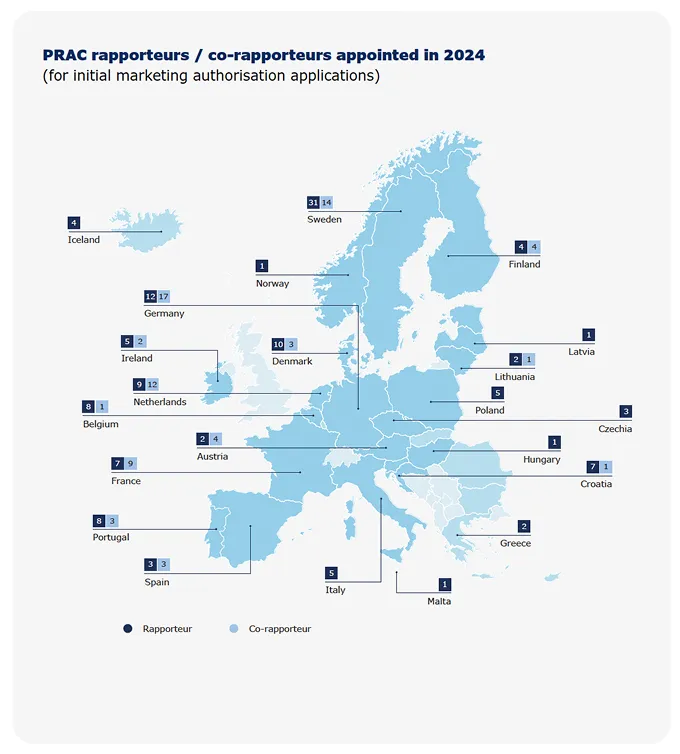
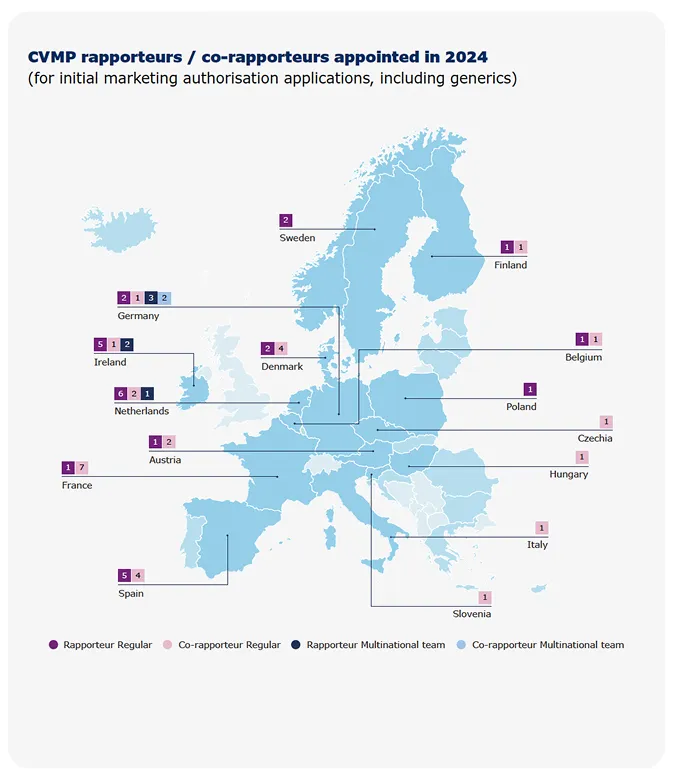
Rapporteurships andco-rapporteurships
The assessment of a medicine by EMA’s scientific committees is carried out by a rapporteur and a co-rapporteur, who prepare the assessment reports and lead discussions in the committees. The appointment is made on the basis of the best possible expertise for the particular product. Rapporteurs work through assessment procedures and take the lead in evaluating any new information on the medicine that may become available.
EMA and its regulatory network partners run a scheme to enable multinational teams to assess applications for human and veterinary medicines. The aim is to mobilise the best expertise for medicines evaluation, regardless of where experts are based. The concept enables rapporteurs and co-rapporteurs for EMA’s scientific committees to include experts from other Member States in their assessment teams. This helps to optimise resource use across the regulatory network and encourage cross-border fertilisation of scientific expertise.