Antimicrobial resistance
Antimicrobial resistance (AMR) is an increasingly serious public health threat. It threatens the effective treatment of an ever-increasing range of infections caused by bacteria and other microorganisms.
In 2019, EMA contributed to the global fight against AMR by:
- supporting the development of new antimicrobial agents;
- collecting data on consumption of veterinary antimicrobials;
- encouraging and advising on responsible use of antimicrobials.
SUPPORTING THE DEVELOPMENT OF NEW ANTIMICROBIAL AGENTS
EMA supports the development of new medicines and treatment approaches, especially for patients with multi-drug resistant bacteria. In 2019, two new antibacterial agents received a positive opinion from CHMP: Quofenix (delafloxacin) is an antibiotic used in adults to treat bacterial infections of the skin and underlying tissues; Recarbrio (Imipenem/ cilastatin/relebactam) is intended for the treatment of infections due to certain bacteria (aerobic Gramnegative bacteria).
EARLY DIALOGUE WITH MEDICINE DEVELOPERS
Fostering the development of new medicines to treat resistant infections is one of the main pillars in the fight against the global threat of AMR and is a high priority for EMA and the EU medicines regulatory network.
In May 2019, EMA opened the Innovation Task Force (ITF), its platform for early dialogue, to all medicine developers working on medicines for the treatment or prevention of life-threatening microbial infections to help strengthen the industry’s drug-development pipeline for new antimicrobials. Based on the initial experience with product-specific discussions in this new framework, the initiative facilitates greater interaction between developers and the regulator streamlining and optimising respective drug developments in the AMR field and, as such, is facilitating their way to the patients. Promising drug candidates will have the opportunity for further interactions through the various development support measures offered by EMA.
A COMMON APPROACH FOR NEW ANTIBIOTIC DEVELOPMENT
EMA also published a revision of its guideline on the evaluation of human medicines indicated for the treatment of bacterial infections. The revision is the result of cooperation between the Agency and its international partners in the United States and Japan which aims to align the data requirements as much as possible between the three regions. The revised guideline was published for a sixmonth public consultation in July 2019.
EMA, the US FDA and the Japanese PMDA continued their cooperation throughout the year. In their fourth tripartite meeting, held on 24 and 25 September 2019 in Tokyo, the discussions revolved around the design of clinical trials that could meet the data requirements of different regulators. For the first time, the meeting also included a discussion on antifungal agents, an area increasingly affected by AMR and where clinical development programmes can be challenging.
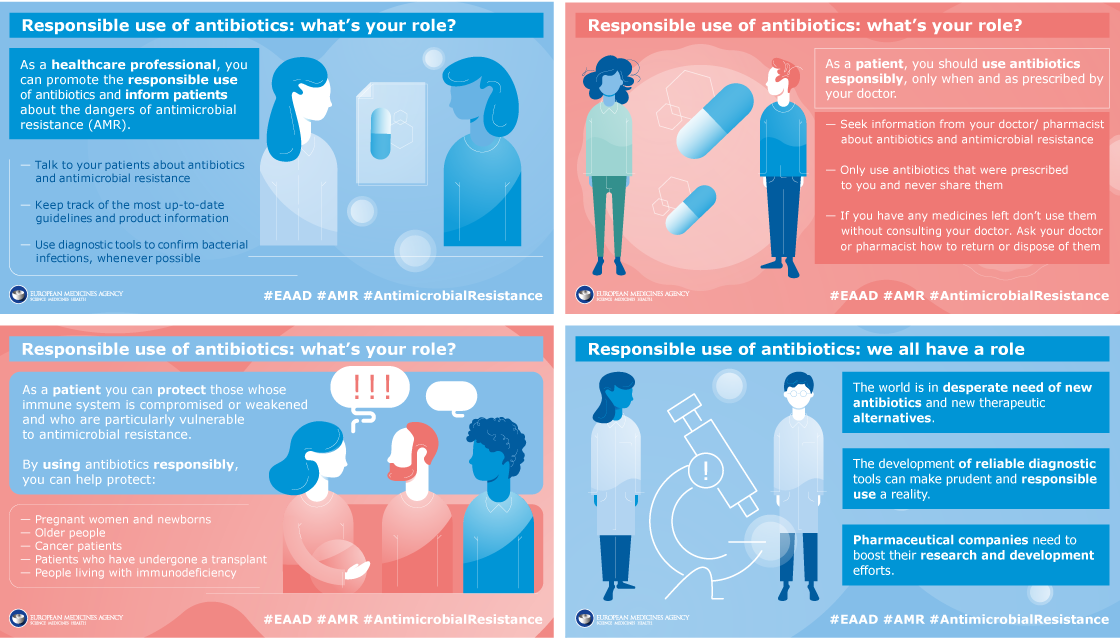
What does responsible use mean for you - European Antibiotic Awareness Day 2019
To mark European Antibiotic Awareness Day in 2019, EMA launched a social media campaign to highlight the importance of using antibiotics responsibly. A set of info-cards focused on what responsible use means for patients, healthcare professionals, veterinarians, health leaders and the pharmaceutical industry.
DATA SHOW DECREASE IN THE SALES OF VETERINARY ANTIMICROBIALS
The 9th European Surveillance of Veterinary Antimicrobial Consumption (ESVAC) report, published by EMA in October 2019 and reporting 2017 sales data for 31 participating countries, showed encouraging results on the sales of veterinary antibiotics. For the 25 countries that contributed data between 2011 and 2017, the sales of antibiotics for animal use decreased overall by 32%. The report is used by risk assessors and risk managers in Member States as a reference for antimicrobial policies and for guidance on the responsible use of antimicrobials.
While the situation across EU Member States remains variable, the report gives reason for optimism that the substantial reduction in the sales of antimicrobials for food-producing species observed in some countries indicates the potential for a similar decrease in others, especially in those where consumption is still high.
The ESVAC network has grown from nine countries reporting data for its first report published in 2011 to 31 countries from the European Economic Area and Switzerland providing data from 2017 in the report published in 2019.
Reduction in sales is the result of combined efforts of veterinarians, farmers, other actors in the livestock sector, EU Member States, the European Commission and EMA. National campaigns for prudent use of antibiotics in animals, sales targets and restriction of use of some antimicrobials in food-producing animals, as well as EU guidance, have helped to reduce the sales of veterinary antimicrobials across Europe.
RESPONSIBLE USE OF ANTIBIOTICS IN ANIMALS PROTECTS PEOPLE AND ANIMALS
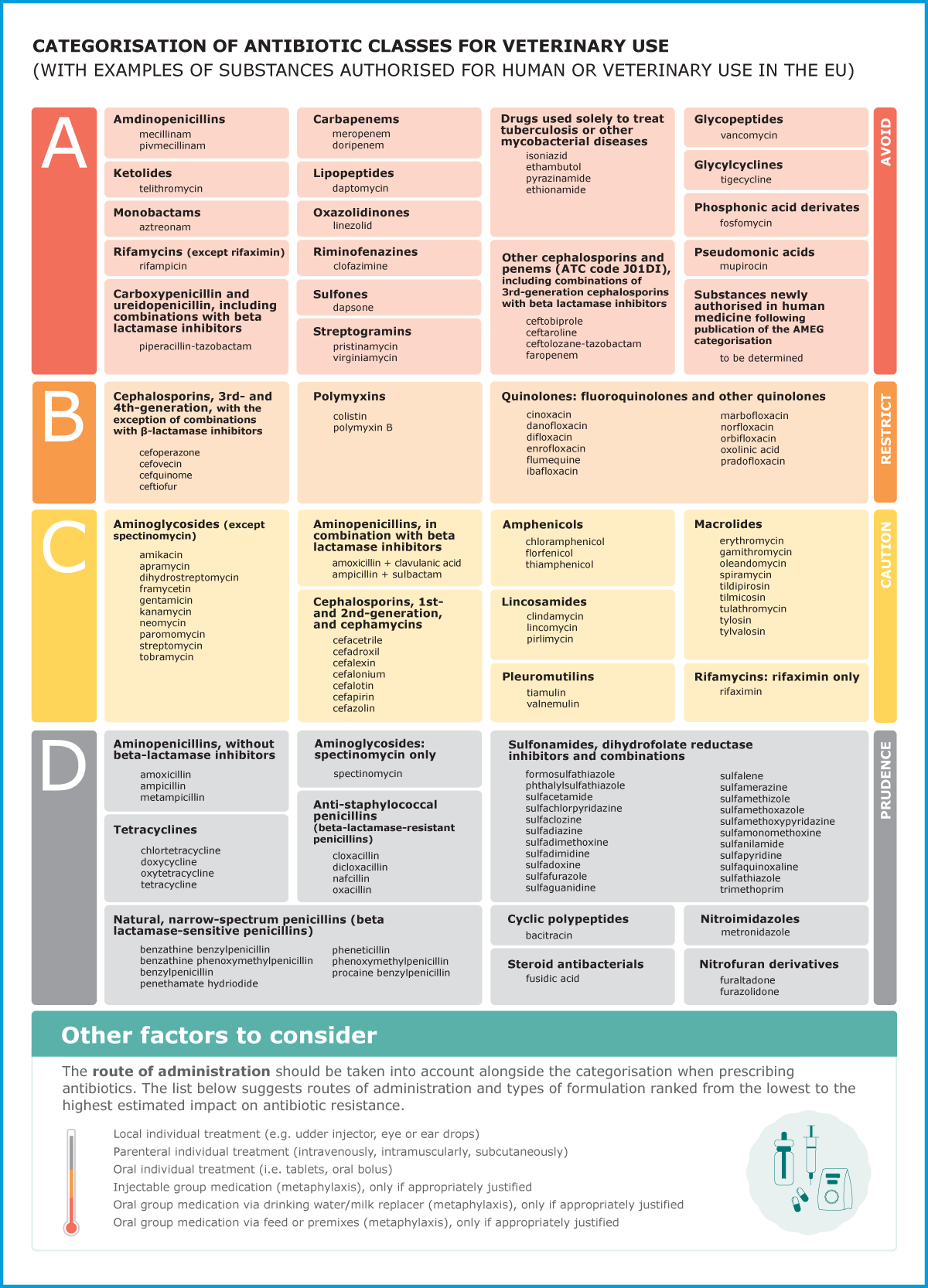
In 2019, EMA launched a public consultation on its updated scientific advice on the categorisation of antimicrobials. The scientific advice ranks antibiotics by considering both the risk that their use in animals causes to public health through the possible development of AMR and the need to use them in veterinary medicine.
The update took into account the experience gained since the initial publication of the categorisation of antimicrobials in 2014. It now addresses all classes of antibiotics, including those classified as Critically Important Antimicrobials (CIA) for human health by the World Health Organization. The classification comprises four categories, from A to D: Avoid (A), Restrict (B), Caution (C) and Prudence (D).
Veterinarians are encouraged to check and consider EMA’s updated scientific advice on the categorisation of antibiotics when prescribing these medicines for animals in their care. The categorisation can also be used as a tool for the preparation of treatment guidelines.
A JOINT ENDEAVOUR
The above-mentioned scientific advice was prepared by the Antimicrobial Advice Ad Hoc Expert Group (AMEG), comprising representatives and experts from the CVMP and the CVMP’s Antimicrobials Working Party, the CHMP and the CHMP’s Infectious Diseases Working Party, the EFSA, the ECDC, and the Joint Interagency Antimicrobial Consumption and Resistance Analysis (JIACRA) working group. It was adopted by both CVMP and CHMP in December 2019 in line with EMA support for a ‘One Health’ approach that promotes close and integrated cooperation between human and veterinary medicine.