Hero image
Image

Pre heading
CHAPTER 2 - KEY FIGURES IN 2019
Network, stakeholders, administration and communication
Assembly area
Heading
Rich text
The European medicines regulatory network is the basis for EMA’s success. The pool of over 4,000 experts made available by the national competent authorities in the Member States enable EMA’s public health mandate by providing the best available scientific expertise for the regulation of medicines in the EU. Here are some key figures on the network, staffing and EMA’s communication and interactions with stakeholders. For more detailed information, download the full annual report 2019 (PDF version).
Heading
Rich text
EUROPEAN MEDICINES REGULATORY NETWORK
First column content
Image

Second column content
Image

Heading
Rich text
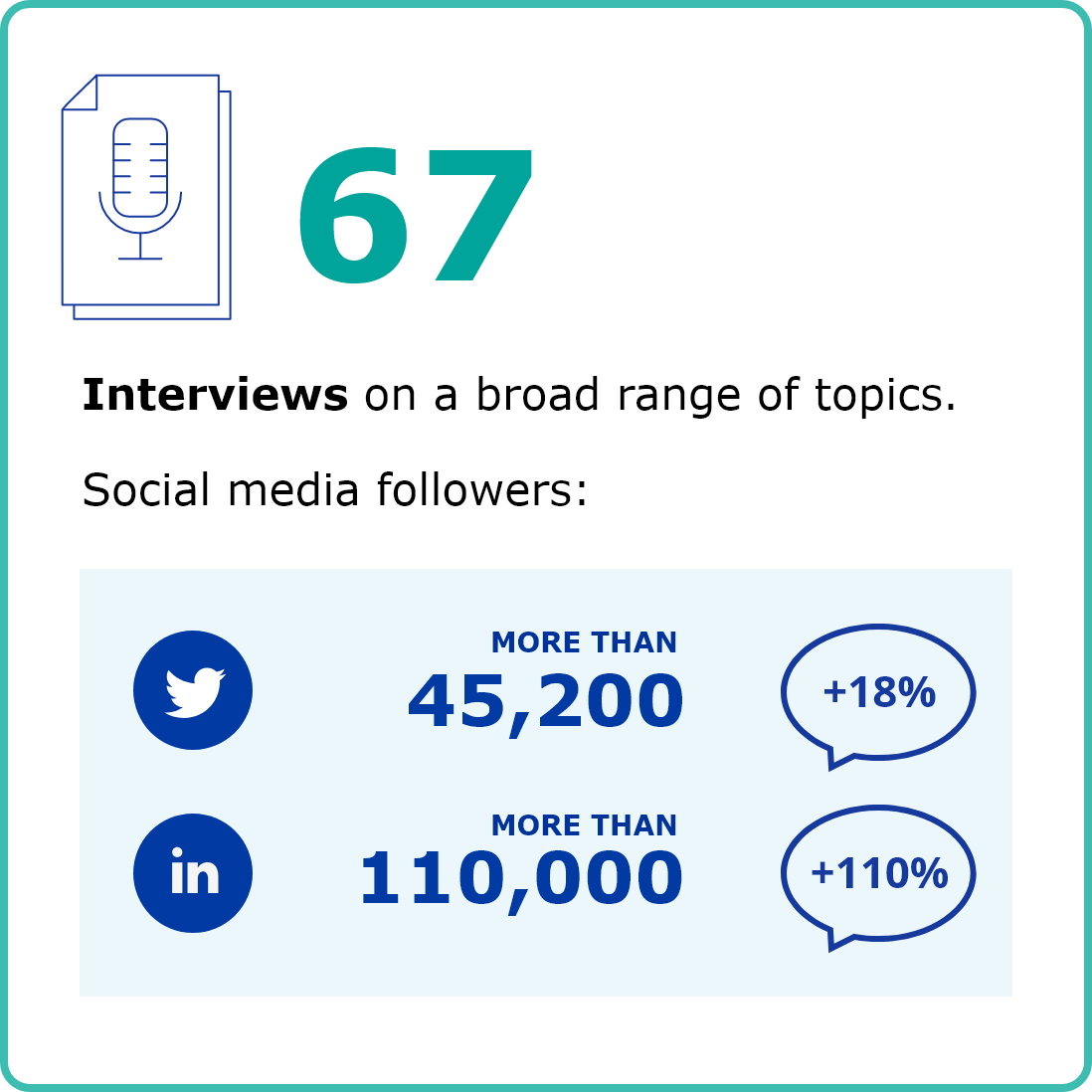
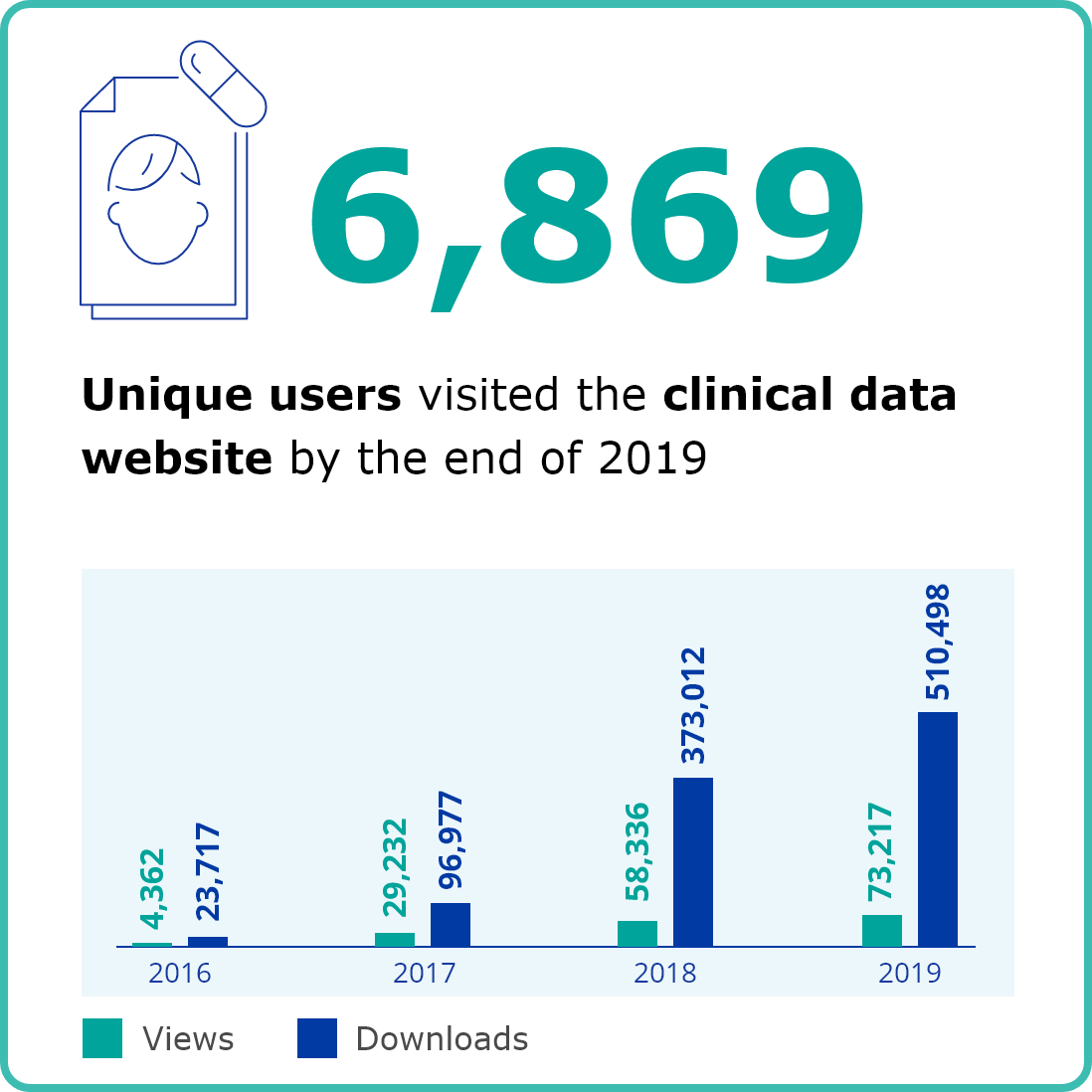
COMMUNICATION & STAKEHOLDERS
First column content
Image
Image

Second column content
Image
Image
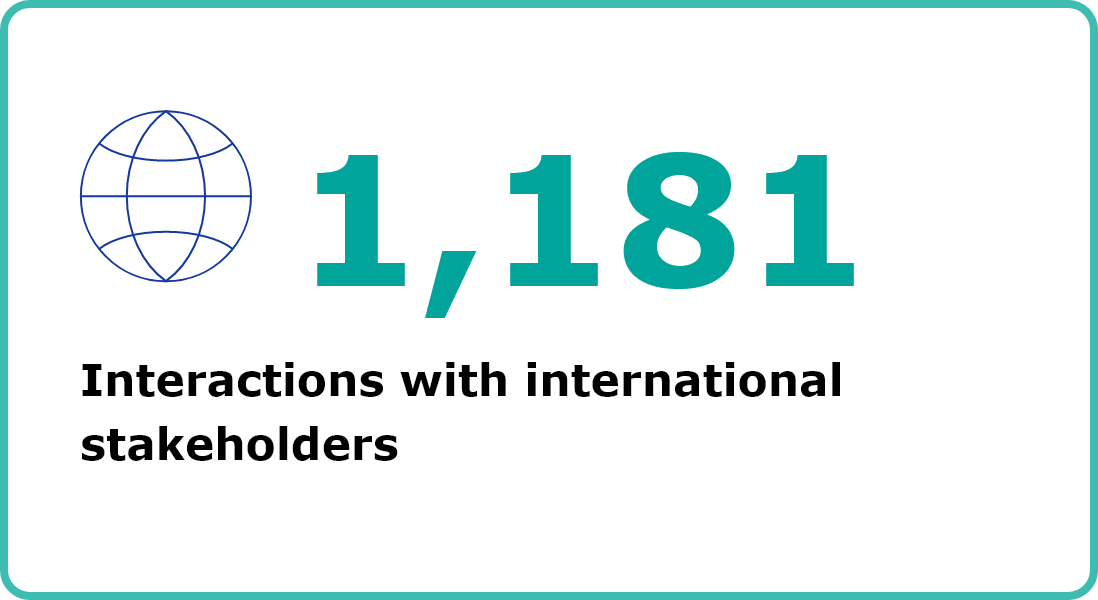
Heading
Rich text
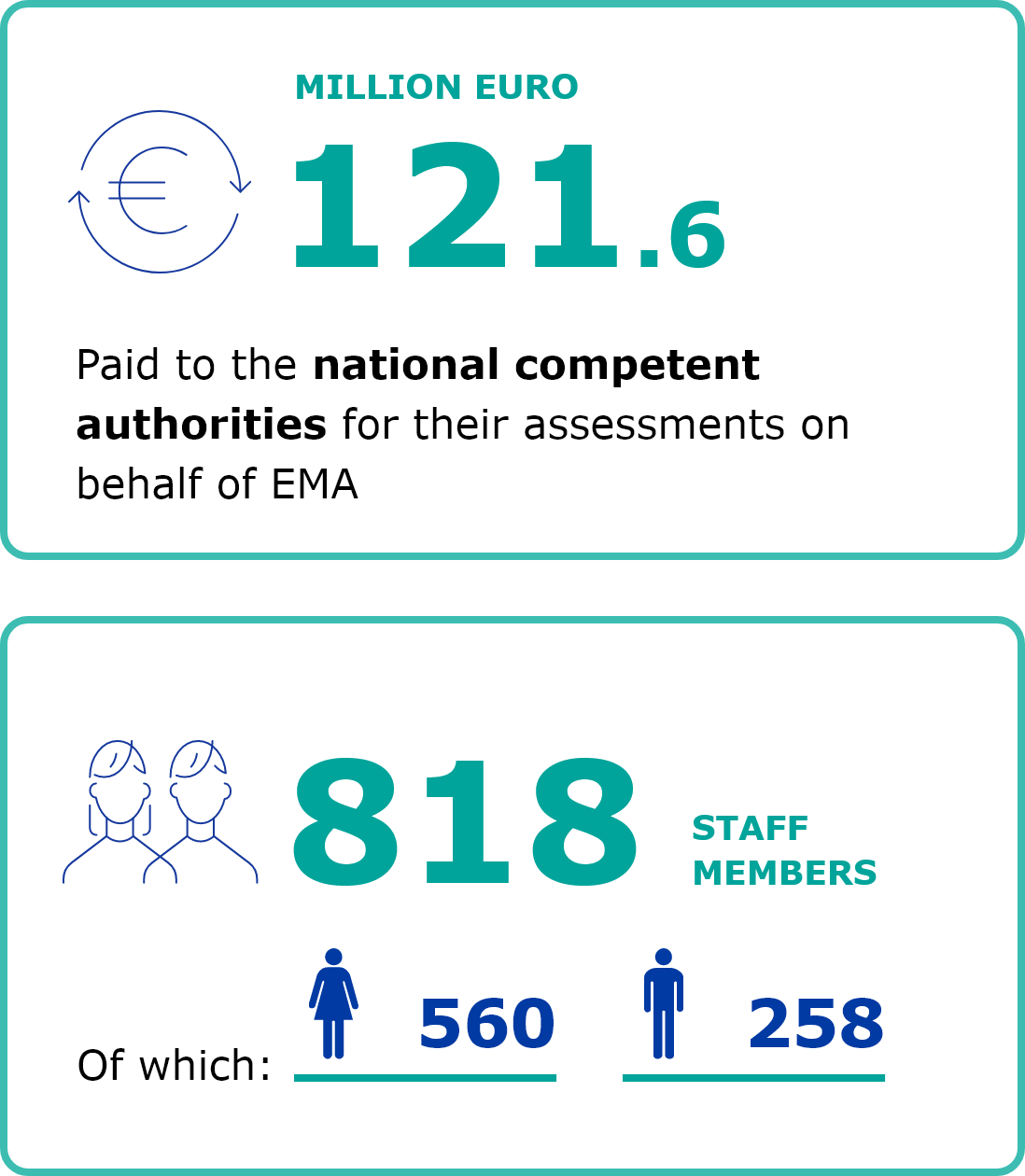
ADMINISTRATIVE ASPECTS
First column content
Image
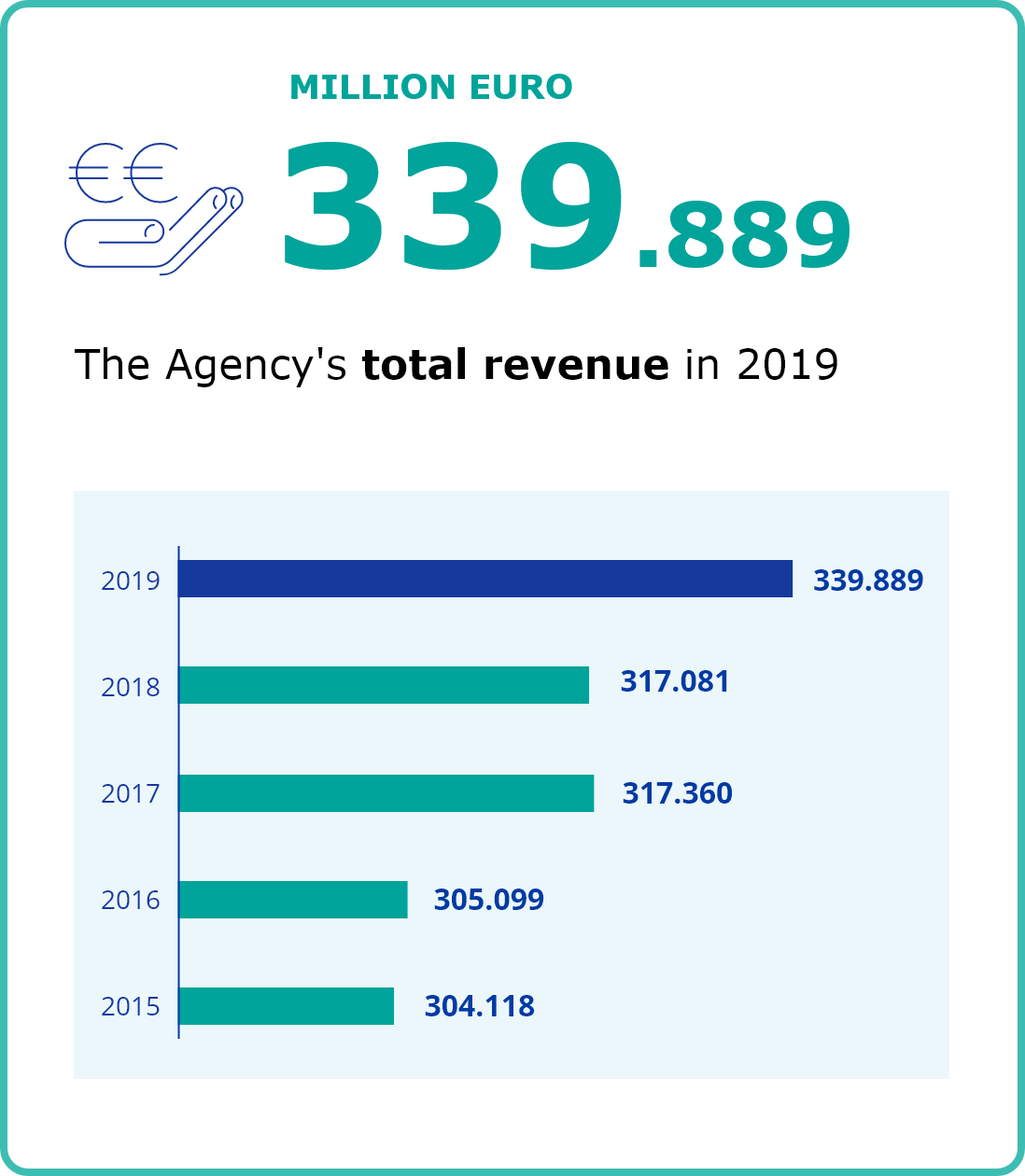
Second column content
Image