This page lists questions that stakeholders, particularly marketing authorisation holders (MAHs), may have on Article 30 referral procedures.
It provides an overview of the European medicines Agency's practical and operational aspects with regards to handling of Article 30 referral procedures. Revised topics are marked 'New' or 'Rev.' with the relevant date upon publication.
A PDF version of these questions and answers is available:
These questions and answers are for guidance only, and should be read in conjunction with the rules governing medicinal products in the European Union, Volume 2A, chapter 3, notice to applicants. Marketing authorisation holders (MAHs) must in all cases comply with the requirement of EU legislation.
Initiation of Article 30 referral
An Article 30 'harmonisation' referral procedure follows the provisions of Article 30 of Directive 2001/83/EC.
It applies when divergent decisions have been adopted by the Member States (MSs) concerning the authorisation of a nationally authorised medicinal product, in order to promote harmonisation of authorisations. It also applies when divergent decisions have been adopted by MSs concerning the suspension or revocation of a medicinal product.
The procedure for an Article 30 referral procedure is laid down in Articles 32, 33 and 34 of Directive 2001/83/EC.
References:
An Article 30(1) referral procedure may be initiated when divergent decisions have been adopted by Member States (MSs) concerning the authorisation (e.g. different indications, posology, contraindications or warnings), suspension or revocation of a particular medicinal product. This procedure can be initiated by a MS, the European Commission (EC) or a marketing authorisation holder (MAH)/applicant where divergent decisions have been taken for an application submitted in two or more MSs.
An Article 30(2) referral procedure may be initiated for the same reasons in order to promote harmonisation of authorisations for medicinal products authorised in the European Union (EU). A list of products proposed for harmonisation is drafted each year by the Co?ordination Group for Mutual Recognition and Decentralised Procedures (CMDh), taking into account the proposals from all MSs, and forwarded to European Commission (EC). The Article 30(2) referral procedure may then be initiated for products on this list by the EC or a MS, in agreement with the Agency and taking into account the views of interested parties.
References:
An Article 30 referral procedure can be initiated by the national competent authorities (NCAs) in Member States (MSs), the European Commission (EC) or by the marketing authorisation holder (MAH)/applicant.
The initiator of the referral procedure refers the matter to the Committee for Medicinal Products for Human Use (CHMP) by circulating a notification form to the Agency, all MSs and the EC.
The notification form will identify the areas of divergence amongst the national decisions and the question(s) sent to the CHMP for consideration.
The MAH/applicant can request a pre-referral meeting with the Agency, this is particularly advisable in case he intends to initiate a referral under Article 30(1).
References:
Any variation(s) submitted but not approved (pending) should be mentioned at the pre-referral stage as well as in the referral submission, informing the Agency and (co-)rapporteurs. Submitted variations will be taken into account during the referral only if approved in at least one Member State (MS) (including Iceland and Norway).
Although the Article 30 referral is independent from any paediatric submission or paediatric work sharing exercises, Periodic Safety Update Report (PSUR) submissions and annual re-assessments, these should be highlighted clearly at the pre-referral stage as well as in the marketing authorisation holder's (MAH) submission(s).
For Article 30 referral procedures, only the concerned medicinal product for which divergent decisions have been adopted in Member States (MSs) concerning its authorisation, suspension or revocation shall be included.
The marketing authorisation holder (MAH) will be requested to provide a list of the (invented) names of the medicinal product, the name of the company in the MSs where the product is authorised, strength(s), pharmaceutical form(s), route of administration(s), content (as applicable) in the respective MSs of the European Economic Area (EEA). This will be checked with the national competent authorities (NCAs) of the MSs.
Parallel Article 30 referral may be considered for medicinal products that include different salt forms or pharmaceutical alternatives that may have an impact on bioavailability/administration schedules, or where single active substances and their fixed combinations are being considered.
To facilitate the exchange of information prior to the start and during the procedure, the marketing authorisation holder (MAH)/applicant should inform the Agency of the designated contact person for the Article 30 referral procedure.
The MAH/applicant may, if they wish, be represented by another party (e.g. a consultant), who will be the contact person for the procedure. In this case it must inform the procedure manager identified in the letter notifying the procedure initiation.
All documentation concerning the Article 30 referral procedure will be sent to the contact person only. The contact details of the person should be clearly stated (name, address, phone and fax number and email address) in the letter of representation.
It is the responsibility of the MAH/applicant to notify the Agency of any change that might affect the validity of the letter of representation as soon as possible (e.g. in case of a change of the contact person), and to provide a relevant revised letter of representation.
All documentation concerning the Article 30 referral procedure will be sent to the contact person only.
A brief summary of the issue will be discussed at the upcoming Committee for Medicinal Products for Human Use (CHMP) plenary meeting and will be included in the agenda published at the beginning of the meeting.
The start of the procedure will be announced as part of the CHMP meeting highlights, which will be published on the next working day following the CHMP meeting during which the matter is considered.
The announcement will specify the concerns under consideration.
Reference:
The public announcement on the Agency's website will include information related to the start of procedure.
The letter notifying the marketing authorisation holder (MAH) of the procedure initiation will include the name and contact details of the Agency's dedicated procedure manager who will be the primary contact point during the procedure, and the e-mail address of the product-shared mailbox, which should always be copied in all correspondence with the Agency. It will be sent to the MAH together with the notification triggering the procedure, the timetable and the list of questions (if applicable, i.e. the procedure was not triggered by the MAH) adopted by the Committee for Medicinal Products for Human Use (CHMP).
Fees are only payable for referral procedures under Article 30 of Directive 2001/83/EC which have been initiated by the marketing authorisation holder (MAH).
References:
The marketing authorisation holder (MAH) will be requested to submit information relevant for the assessment.
This is an opportunity given to the MAH to present written or oral explanations to the Committee for Medicinal Products for Human Use (CHMP) within a time limit(s) as specified in the procedure timetable before an opinion is issued by the CHMP.
In the case where divergent decisions have been adopted by the Member States (MSs) concerning the authorisation of a medicinal product, the MAH should also submit a proposal for a harmonised summary of product characteristics (SmPC), labelling and package leaflet (PL) within the time limit(s) as specified in the procedure timetable.
The MAH will be informed of the start of the procedure and during the procedure on how and when to submit data (please refer to Questions 8, 11 and 14).
Regardless of whether or not the marketing authorisation holder presents their explanations to the CHMP, an opinion will be issued by the CHMP, in any case, applicable to all marketing authorisations concerned by the procedure.
At the start of the procedure the data considered to be necessary for the assessment will be identified in a list of questions for submission within the specified deadline as indicated in the timetable (please refer to Question 8, 14 and 17).
The Committee for Medicinal Products for Human Use (CHMP) may also collect additional data through a list of outstanding issues and/or in an oral explanation in accordance with an extended timetable.
The assessment of data within the Article 30 referral procedure is the responsibility of the Committee for Medicinal Products for Human Use (CHMP). At the start of the procedure, the CHMP appoints a rapporteur and co-rapporteur(s) who will perform the assessment of all data collected within the agreed timelines.
The assessment of all the available data will result in the CHMP adopting an opinion on the issue reviewed.
The Committee for Medicinal Products for Human Use (CHMP) (co-)rapporteurs for an Article 30 referral procedure is appointed by the CHMP Chairperson from amongst the members or alternates (hereafter referred to as CHMP members), approximately 3 months before the start of the Article 30 referral procedure.
If the procedure was triggered by a Member State (MS), the CHMP member of that MS will be appointed as Rapporteur, if not the rapporteurship will be opened to all members, as will the co-rapporteurship.
The CHMP Chairperson will endeavour to apply the criteria of best available expertise to be taken into account for the appointment of the (co-)rapporteurs for each procedure.
References:
During the assessment
Marketing authorisation holder(s) (MAHs) are requested to submit to the Agency and all Committee for Medicinal Products for Human Use (CHMP) members all available evidence to support the Article 30 referral procedure.
Referral procedures triggered by a Member State or the European Commission
The MAHs should submit their responses to the list of questions (LoQ) as follows:
- The data should be presented electronically according to the electronic Common Technical Document (eCTD)/CTD format and accompanied by a signed cover letter and a written summary of each question.
- The must make clear reference to the procedure number and the Agency's procedure manager should always be put in copy.
- The written summary answering each question should follow the numbering as per the CHMP list of questions/CHMP list of outstanding issues. Please note that supportive data to the responses submitted (e.g. study reports, literature data, risk management plan) are expected to be provided together with a summary of those data as per the modular structure of the CTD format.
- If the procedure was triggered to resolve divergences in the product information (e.g. different indications, posology, contraindications or warnings), the MAH should provide:
- a proposal of harmonised Summary of product characteristics, labelling and package leaflet (in Word format);
- a description the divergences across Member States for each section of the Summary of Product Characteristics;
- the rational with supportive evidence for the proposed harmonised wording. When applicable, this should include a justification, supported by evidence as to why it is considered appropriate not to include wording currently existing in a MS in the harmonised PI.
Referral procedures triggered by a marketing authorisation holder
- In these referrals, the procedure starts with the evaluation of the data submitted by the MAH.
It is left to the MAH's discretion to submit the relevant documentation necessary for the evaluation of the matter referred to justify the proposed harmonised product information, with a particular focus on the resolution of divergences. The same principles as described above are recommended to be followed. This should be accompanied by an expert report/overviews, which have been updated to reflect the current regulatory status. In all cases, data submitted should be accompanied by an overall summary of its content and should make reference to the specific CHMP question being addressed (as per CHMP published list of questions/list of outstanding issues numbering). A listing of all studies (pre-clinical, clinical, post-marketing studies, etc.) and literature referred to in the responses is also strongly recommended.
Published data can be presented as supportive documentation in response to a specific question if no other data is available.
It should be noted that the responsibility for the quality of the submitted documentation lies with the MAH and is crucial to the overall assessment. All submissions are expected to be submitted in English and electronically only (please refer to Question 15).
Reference:
Responses from the marketing authorisation holder (MAH) should be submitted to the Agency within the timeline specified in timetable enclosed to the letter notifying the MAH of the procedure initiation.
All submissions for referral procedures should be sent via the eSubmission Gateway or eSubmission Web Client. These portals send automated acknowledgement of receipt of submission, or of failed submission if an error occurred. The Agency no longer accepts submissions on CD-ROM or DVD.
Please note that submissions for nationally authorised products (NAPs), are not available via the Common Repository and should be sent separately to each NCA. For all referral submissions related to NAPs, the Agency strongly recommends using the electronic Common Technical Document (eCTD) or Non-eCTD electronic Submissions (Nees) formats.
For all types of submissions, responses should be presented in the modular format.
Documentation - Recommended folder structure
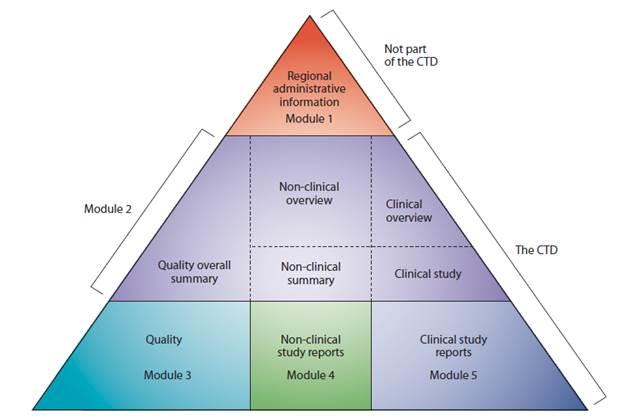
Documentation can be included in respective modules following the CTD location as referenced in the above folder structure – e.g. as following:
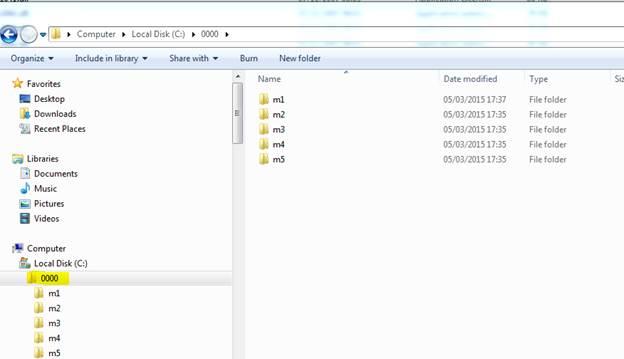
- Root folder should be 4 digits (between 0000-9999), e.g. 'submission 0000'. See screenshot below
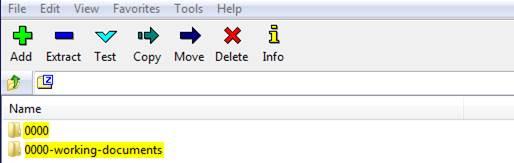
Any working documents (for example: documents in Word format) should be outside the root submission folder, e.g. as following:
More information on the required naming conventions and file formats can be found in detailed examples of filenames for different application types and in the eSubmission gateway web client - guidance for applicants. For more information please refer to eSubmission website.
There is no need to send any separate paper cover letters for these submissions, as the cover letter will be in the relevant part of eCTD/CTD module 1 in PDF format.
Should you have any questions regarding your submission, please contact us via email: referralsubmission@ema.europa.eu, for any technical issues contact esubmission@ema.europa.eu.
Submissions from the marketing authorisation holder (MAH) are provided directly to the Committee for Medicinal Products for Human Use (CHMP) (co-)rapporteurs to be considered in their assessment.
All information gathered will be assessed within an agreed timeframe (please refer to Question 17). The assessment report(s) prepared by the CHMP (co-)rapporteurs will reflect all data reviewed and considered relevant for the assessment.
The CHMP may in some cases require input from individual experts to advise it on specific questions in relation to the assessment.
The CHMP (co-)rapporteur assessment report(s) will be circulated to the CHMP members for comments.
Please note that the timelines below are provided for guidance purposes only and they refer to active days, which correspond to the time the Committee for Medicinal Products for Human Use (CHMP) takes to assess the data provided.
The timetable for the procedure when triggered by a Member State (MS) or the European Commission (EC) is as follows:
Article 30 referral initiated by a Member State or the European Commission - Timetable for the assessment | Day |
|---|---|
Notification of a referral to the CHMP/Agency Secretariat | Day 0 |
Discussion at the first meeting of the CHMP following receipt of the notification:
| Day 1 |
Preparation and submission of written explanations by the MAH/applicant in response to the CHMP list of questions | Clock Stop |
Re-start of the procedure in accordance with procedural timetables | Clock re-start |
Circulation of the CHMP (co-)rapporteur's assessment report(s) on the MAH's/applicant written responses and the proposed SmPC/labelling/PL, if applicable | Day 20 |
Comments in writing from CHMP members on the CHMP (co-)rapporteur's assessment reports and proposed SmPC/labelling/PL, if applicable | Day 25 |
Discussion at the CHMP meeting:
| Day 30 |
Preparation and submission of written and/or of oral explanations if applicable | Clock Stop |
Re-start of the procedure following submission of written explanations (in accordance with the procedural timetables) or at the time of oral explanations | Clock re-start Day 31 |
Discussion at the CHMP meeting:
| Day 60 |
The timetable for the procedure when triggered by a MAH is as follows:
Article 30 referral initiated by a MAH - Timetable for the assessment | Day |
|---|---|
Notification of a referral to the CHMP/Agency Secretariat Agency to liaise with MAH to ensure that the relevant documentation is submitted to the CHMP/Agency secretariat | Day 0 |
Discussion at the first meeting of the CHMP following receipt of the notification (provided that the relevant documentation has been submitted by the MAH in advance of the start of the procedure):
| Day 1 |
Circulation of the CHMP (co-)rapporteur's assessment report(s) on the MAH's submitted documentation and the proposed SmPC/labelling/PL, if applicable | Day 20 |
Comments in writing from CHMP members on the CHMP (co-)rapporteur's assessment report(s) and proposed SmPC/labelling/PL, if applicable | Day 25 |
Discussion at the CHMP meeting:
| Day 30 |
Preparation and submission of written explanations by the MAH in response to the CHMP list of questions | Clock Stop |
Re-start of the procedure in accordance with the procedural timetables | Clock re-start Day 31 |
Circulation of the CHMP (co-)rapporteur's assessment report(s) on the MAH's written responses and the proposed SmPC/labelling/PL, if applicable | Day 51 |
Comments in writing from CHMP members on the CHMP (co)-rapporteurs assessment report(s) and proposed SmPC/labelling/PL, if applicable | Day 55 |
Discussion at the CHMP meeting:
| Day 60 |
The dates to be followed in accordance to the above timetable for each month can be found in the procedural timetables.
The CHMP may extend the time limit of 60 days to 150 days (which will include clock-stops) to allow for the assessment of further data provided as answers to the CHMP list of outstanding issues, or in an oral explanation, or in cases where the CHMP requires input from experts to support the CHMP opinion.
As a general rule, a clock-stop of up to one month will apply. For an extension of the clock-stop adopted by the CMHP, the MAH should send a justified request to the Agency for agreement by the CHMP. The letter specifying the length of the requested extension should be addressed to the CHMP Chairperson, signed and sent electronically to the EMA procedure manager. In preparing the justification, the MAH should consider the issue under consideration and the impact the extension may have. The CHMP will consider the request, and if agreed, an extended timetable may be adopted.
The marketing authorisation holder (MAH) will be provided with the Committee for Medicinal Products for Human Use (CHMP) (co-)rapporteur's assessment report(s) electronically via Eudralink.
In compliance with article 59(3) of Directive 2001/83/EC (consultation with target patient groups), data should be submitted and assessed to ensure compliance of the package leaflet (PL) with the summary of product characteristics (SmPC), and also to ensure that the way in which the information is set out in the document is accessible to the reader, easy to read (readability) and easy to navigate.
During an Article 30 referral, it is recommended that the results of the consultation with target patient groups are submitted with the responses to the list of outstanding issues (LoOI).
A full user consultation may not be necessary in every case, and a 'bridging study' may be prepared to support the PL, where justified.
References:
The Committee for Medicinal Products for Human Use (CHMP) may decide whether there are issues that also need to be addressed orally by the marketing authorisation holder (MAH)/applicant. The MAH/applicant will be duly informed in advance of the issues to be addressed during an oral explanation.
The MAH/applicant may also make a request to the CHMP to attend an oral explanation. In such a case, the MAH/applicant should send a written request to the CHMP stating the reason(s) and specifying the issue(s) to be addressed during the oral explanation. The CHMP will take due account of the request and will decide whether the oral explanation should be held.
The oral explanation should take place during the assessment phase and after the receipt of the CHMP (co-)rapporteur's assessment report(s). See further detailed information on .
If the marketing authorisation (MA) for a nationally authorised product is withdrawn or transferred during the referral procedure, the former marketing authorisation holder (MAH) should inform the Agency. The Agency will then liaise with the national competent authority (NCA) of the Members State (MS) concerned.
Following confirmation by the NCA of the withdrawal of the MA, the Agency will inform the former MAH that the specific product will no longer be included in the ongoing referral procedure.
Following confirmation by the NCA of the transfer of a MA, the Agency will inform the transferee that they are included in the referral procedure.
If the name of a nationally authorised product or if the name and/or address of a marketing authorisation holder (MAH) changes during the referral procedure, the marketing authorisation holder (MAH) should inform the Agency. The Agency will then liaise with the national competent authority (NCA) of the Member State (MS) concerned. Following confirmation by the NCA of the change, the Agency will inform the MAH that the change has been noted.
Committee for Medicinal Products for Human Use (CHMP) opinion
The Committee for Medicinal Products for Human Use (CHMP) will issue an opinion on the matter referred under Article 30 within 60 days of the start date of the procedure. The CHMP may extend that period up to 150 days, to take into account all available data as well as any issues addressed by the marketing authorisation holder (MAH) during an oral explanation and/or input from experts (if any) before issuing an opinion.
The CHMP opinion will usually be adopted on the last day of the CHMP's plenary meeting.
The Committee for Medicinal Products for Human Use (CHMP) opinion on an Article 30 referral procedure may be that:
- the summary of the product characteristic (SmPC) as proposed by the applicant should be amended, or the marketing authorisation(s) (MA(s)) should be varied, as applicable;
- the MA(s) should be subject to certain conditions;
- the application does not satisfy the criteria for authorisation, or the MA(s) should be suspended or revoked, as applicable.
Where the product information is to be amended, the CHMP opinion will include the entire revised harmonised product information texts (i.e. summary of product characteristics (SmPC), labelling or package leaflet (PL).
Where the MA should be subject to certain conditions or is to be suspended with condition(s) for lifting the suspension of the marketing authorisation(s), these can include, but are not limited to, requesting the marketing authorisation holder to conduct a post-authorisation safety/efficacy study. The assessment of the fulfilment of the condition(s) to the marketing authorisation(s) will be the responsibility of the Member States, under the lead of the reference Member State (RMS) unless otherwise stated.
The CHMP opinion can be adopted by consensus or by majority vote. In the event of an adoption by majority, the divergent positions of the concerned CHMP members will be appended to the opinion.
The Committee for Medicinal Products for Human Use (CHMP) opinion will include:
- a cover page in which the adopted opinion is outlined together with the voting outcome of CHMP;
- a listing of all products/applications concerned, including the names of all identified products authorised nationally including via the mutual recognition/decentralised procedures, their respective marketing authorisation holders (MAHs)/applicants in each Member State;
- the scientific grounds and explanations for the CHMP opinion;
- the harmonised summary of product characteristics (SmPC) and/or the labelling or package leaflet (PL), if applicable;
- the conditions or restrictions imposed on the marketing authorisation(s), if applicable;
- the CHMP members' divergent views, in case the opinion is adopted by majority;
- the CHMP assessment report on the evaluation performed and the conclusion of the CHMP that led to the adoption of the opinion based on all the data gathered;
- the Direct Healthcare Professional Communication (DHPC) and communication plan as agreed by CHMP, if applicable.
A brief outcome of the Committee for Medicinal Products for Human Use (CHMP) opinion will be included in the CHMP meeting highlights that are released on the Friday of the CHMP plenary meeting week, together with a summary of the CHMP conclusions in the format of a Question & Answers document and with the adopted product information, if applicable.
The CHMP opinion will be published on the procedure webpage following the adoption of the European Commission Decision (please refer to Question 31).
Reference:
The designated contact person representing the marketing authorisation holder (MAH) (please refer to Question 6) identified at the start of the procedure, will receive the Committee for Medicinal Products for Human Use (CHMP) opinion and assessment report during the week following the adoption of the CHMP opinion.
The marketing authorisation holder (MAH)/applicant may, within 15 calendar days of the receipt of the Committee for Medicinal Products for Human Use (CHMP) opinion, notify the Agency in writing of its intention to request a re-examination of the CHMP opinion.
After these 15 calendar days, if the MAH/applicant has not requested a re-examination, the CHMP opinion is considered final and is sent to the European Commission (EC) for the initiation of the decision-making process.
Re-examination
If within 15 days after the receipt of the CHMP Opinion the MAH/applicant has notified the Agency in writing of its intention to request a Procedural advice on the re-examination of CHMP opinions, the Agency will inform the CHMP of the letter of intent received.
The detailed grounds for the re-examination requested should be sent to the Agency within 60 calendar days of receipt of the CHMP opinion. The detailed grounds submitted will determine the scope of the re-examination procedure and may encompass all aspects set out in the CHMP opinion or only certain aspects of it. However, no new data can be presented at this stage of the procedure.
The re-examination procedure will only deal with the aspects of the CHMP opinion identified by the MAH/applicant in the detailed grounds for re-examination. The MAH may request that the CHMP consults a scientific advisory group (SAG) or ad-hoc expert group during the re-examination procedure. In such a case, this request should be made as early as possible, and should be no later than the submission date of the detailed grounds.
New (co-)rapporteurs will be appointed for the re-examination, and within 60 calendar days of receipt of the detailed grounds for re-examination, the CHMP will conclude its assessment of the detailed grounds and adopt a final opinion.
The CHMP final opinion following re-examination is sent to the EC for the initiation of the decision-making process.
The marketing authorisation holder (MAH) will have to provide translations in all EU languages (including Icelandic and Norwegian, if applicable1 of the following annexes to the Committee for Medicinal Products for Human Use (CHMP) opinion:
- listing of nationally authorised products (including via the mutual recognition/decentralised procedures) concerned by the procedure;
- the harmonised summary of product characteristics (SmPC) and/or the labelling and/or package leaflet (PL), if applicable
The Agency will contact the MAH/applicant as early as possible to ensure the smooth running of the process. The translations will have to be provided to the Member States contact points for linguistic check by Day +5 (i.e. 5 days after adoption of the opinion) and copied to the Agency. Member states may send linguistic comments until Day +19. The MAH should send the translations amended accordingly together with the completed Practical information on translations for referral procedures (human) 2 to the Agency by Day +22.
See detailed information on the Practical information on translations for referral procedures (human).
1 If authorised in Iceland and Norway.
Following the adoption of the Committee for Medicinal Products for Human Use (CHMP) opinion, the Agency together with the marketing authorisation holder (MAH) and national competent authorities (NCAs) in the Member States (MSs) will finalise the translations and will send these to the European Commission (EC).
The EC will then start the decision-making process leading to the adoption of a binding decision addressed to the MSs and notified to the MAH/applicant.
See detailed information on the decision-making process.
See the Co?ordination Group for Mutual Recognition and Decentralised Procedures (CMDh) recommendation for implementation of Commission Decisions.
Around one week following the adoption of the European Commission (EC) decision the Committee for Medicinal Products for Human Use (CHMP) assessment report, in English only, will be published on the procedure webpage. Within four weeks of the adoption of the EC decision, the CHMP opinion with its annexes and the Question & Answers document in all EU languages will also be published on procedure webpage on the Agency's website, which will be updated to reflect the date of the EC decision.
Reference: