This page lists questions that stakeholders, particularly marketing-authorisation holders (MAHs), may have on urgent Union procedures.
It provides an overview of the European Medicines Agency's practical and operational aspects with regards to the handling of these procedures. Revised topics are marked 'New' or 'Rev.' on publication.
A PDF version of these questions and answers is available below.
These questions and answers are for guidance only, without prejudice to legal and regulatory interpretation that might be provided in future updates of the rules governing medicinal products in the European Union, volume 2, notice to applicants.
These questions and answers should be read in conjunction with Directive 2001/83/EC, as amended by Directive 2010/84/EU of the European Parliament and of the Council of 15 December 2010, without prejudice to the implementation of the changes resulting from Directive 2012/26/EU of the European Parliament and of the Council of 25 October 2012 amending Directive 2001/83/EC as regards pharmacovigilance.
Initiation of the urgent Union procedure
An urgent Union procedure follows the provisions under Article 107i of Directive 2001/83/EC.
It applies where urgent regulatory action (please refer to Question 2 and Question 3) is considered on the basis of concerns resulting from the evaluation of data from pharmacovigilance activities of an authorised medicinal product(s).
The procedure for an urgent Union procedure under Article 107i is laid down in Articles 107j to 107k of Directive 2001/83/EC.
References:
An urgent Union procedure is automatically initiated when, on the basis of concerns resulting from the evaluation of data from pharmacovigilance activities, a Member State or the European Commission considers one (or more) of the following situations:
- suspending or revoking a marketing authorisation (MA);
- prohibiting the supply of a medicinal product;
- refusing the renewal of a MA;
is informed by the marketing authorisation holder (MAH) that, on the basis of safety concerns, the MAH has interrupted the placing on the market of a medicinal product or has taken action to have a MA withdrawn, or intends to take such action or has not applied for the renewal of a MA.
References:
This procedure can also be triggered when, on the basis of concerns resulting from of the evaluation of data from pharmacovigilance activities, a Member State or the European Commission considers that urgent action is necessary in case of a new contraindication, a reduction in the recommended dose or a restriction to the indications of a medicinal product authorised in more than one EU Member State1.
1 Where in case of a new contraindication, a reduction in the recommended dose or a restriction to the indications of a medicinal product, this procedure is not initiated but the interests of the Union are involved, the procedure under Article 31 of Directive 2001/83/EC shall apply. Please refer to the Questions and answers: Article 31 pharmacovigilance referrals
This procedure can be initiated by competent authorities in the Member States (MSs) or by the European Commission (EC). A marketing authorisation holder (MAH) cannot trigger this procedure.
The initiator of the procedure will circulate a notification to the Agency, all MSs and the EC triggering the urgent Union procedure. The notification will identify the safety concern including a detailed explanation of the issue raised and the regulatory action which is being considered.
The notification will be publicly available at the start of the procedure (please refer to Question 9).
A Member State (MS) may, where urgent action is necessary to protect public health, suspend the marketing authorisation and prohibit the use of the medicinal product concerned on its territory until a definitive decision is adopted.
In this case, the MS informs the European Commission, the Agency and all other MSs, no later than the following working day, of the reasons for its action (please refer to Question 2, Question 3 and Question 6).
All medicinal products affected by the safety issue and with a valid marketing authorisation (MA) in the European Economic Area (EEA) will be included in the urgent Union procedure regardless of whether the MA was granted nationally (including via the mutual recognition and decentralised procedures) or via the centralised procedure. However, in case the safety issue concerns only centrally authorised medicinal product(s), the procedure under Article 20 of Regulation (EC) No 726/20041is initiated instead.
The procedure may concern a specific medicinal product, all medicinal products containing the same active substance (range of medicinal products) or all medicinal products belonging to the same therapeutic class (several active substances concerned).
The marketing authorisation holder(s) cannot choose whether or not to include their medicinal products in an urgent Union procedure. The inclusion of their medicinal products depends on the scope of the procedure that is defined by the safety concern raised in the notification.
Reference:
1Please refer to the questions & answers on practical implementation of Article 20 pharmacovigilance procedure.
The Agency, with input from the competent authorities of the Member States (MSs) within the European Economic Area (EEA) as necessary, will identify the authorised medicinal products concerned by the procedure. All authorised medicinal products concerned are identified using information from the Article 57 database. This will take place at the start of the procedure, and a draft list of the medicinal products identified will be publicly available on the Agency’s website on the specific procedure page (please refer to Question 9).
If it is concluded that the safety issue triggering the urgent Union procedure also concerns other medicinal product(s) (e.g. range of medicinal products, a therapeutic class) than the ones covered by the notification, the Agency can extend the scope of the procedure.
From a procedural view point, only those medicinal products identified at the start of the procedure will be covered by its scope. However, to the extent that other medicinal products affected by the concern but not identified at start of procedure are authorised in the EEA, or subject to future authorisations by the MSs, the concerned MSs should take due consideration of the scientific conclusions of the procedure and apply them to these medicinal products.
Upon receipt of the notification triggering the urgent Union procedure, the Agency, based on the information collected from the Article 57 database and possibly in consultation with the national competent authorities of the Member States (MSs) within the European Economic Area (EEA), will identify in which MS(s) the concerned medicinal product(s) is authorised.
If it is concluded that the scope of the procedure concerns medicinal product(s) authorised in only one MS, an urgent Union procedure will not be initiated, and the safety issue will be handled by the MS concerned.
Once the Urgent procedure is triggered, the safety issue will be discussed at the upcoming Pharmacovigilance Risk Assessment Committee (PRAC) plenary meeting and a brief summary will be included in the agenda published at the beginning of the PRAC meeting.
The start of the procedure will be announced as part of the PRAC meeting highlights, which will be published on the next working day following the PRAC meeting during which the matter is considered.
In certain cases, depending on the urgency of the matter, the announcement may take place earlier.
The announcement will specify the safety issue under urgent consideration and the modalities for the marketing authorisation holders (MAHs), healthcare professionals and the general public to submit to the Agency information relevant to the procedure (please refer to Question 15, Question 19 and Question 21).
The following documents will be published at the time of announcement of the start of the procedure on the Agency’s website on a dedicated page for the procedure:
- announcement of the start of the procedure;
- notification triggering the procedure together with the scientific background;
- draft product list of concerned medicinal products/active substances and MAHs;
- list(s) of questions and timetable adopted by the PRAC;
- form for submission by stakeholders.
Reference:
Whenever possible, MAHs will be informed on the Wednesday before the PRAC meeting of new urgent Union procedure(s) that the PRAC will consider the following week. This communication will be provided for information only.
Following the PRAC meeting, a public announcement on the Agency’s website will include all information related to the start of procedure. In addition, all qualified persons for pharmacovigilance (QPPV) of the medicinal product(s) concerned by the urgent Union procedure identified in the published product listing will be notified electronically (via e-mail/Eudralink) by the Agency. The notification of the procedure initiation to the QPPV will include:
- the name and contact details of the Agency’s procedure manager who will be the contact point throughout the procedure and the address of the product-shared mailbox, which should be copied in all correspondence with the Agency;
- links to the Agency’s page where the relevant documentation is available.
The Agency may release updated information on the website during the procedure and therefore marketing authorisation holder(s) and other interested parties should continuously check the Agency’s website for any relevant updates (please refer to Question 30, Question 36 and Question 40).
The qualified person for pharmacovigilance (QPPV) will, by default, be the contact person and will receive all correspondence from the Agency regarding the pharmacovigilance procedure.
The QPPV may if they wish to, either designate a different contact person within the organisation of the MAH or designate another party to represent the MAH for the procedure. In this case they must inform the EMA procedure assistant via email.
All documentation concerning the urgent Union procedure will be sent to the contact person only. Receipt of any documents by the contact person will be considered to constitute effective receipt by the MAH inter alia for the purposes of calculating the procedural timelines.
All communications with the Agency should be channelled via the contact person only.
The marketing authorisation holders can form a group for the purpose of the procedure (irrespective of group/company affiliation) in order to provide a single consolidated response and/or oral explanations to the questions raised by the Pharmacovigilance Risk Assessment Committee (PRAC) during the procedure. In this case the cover letter accompanying the single consolidated response and/or request for oral explanation should clearly identify the parties responsible for the submission/request.
If a centrally authorised product is involved in an urgent Union procedure, the qualified person for pharmacovigilance (QPPV) will be notified by the Agency of the start of the procedure, as will all other QPPV (please refer to Question 10). The announcement on the Agency's website will also be linked to the EPAR page of the product.
The Agency will levy a fee for an urgent Union procedure under Article 107i of Directive 2001/83/EC.
The share payable by each marketing authorisation holder (MAH) will be calculated by the Agency based on information recorded in the Article 57 database. An advice note will be sent after the start of procedure, to the relevant qualified person(s) for pharmacovigilance (QPPV) in order to ensure the accurate identification of the chargeable units for the medicinal products involved in the procedure. Following the advice note, an invoice will be sent to each MAH..
For MAHs already qualified as a micro-, small or medium-sized enterprise (SME) by the Agency, or those that send a SME declaration within 30 days of the invoice date, the fee will be reduced (small- or medium-sized enterprise) or waived (micro-sized enterprise).
References:
The marketing authorisation holders (MAHs), health care professionals and the general public have the right to submit information relevant to the safety issue under review within the urgent Union procedure.
This is an opportunity given by the provisions of the pharmacovigilance legislation for data to be submitted by all stakeholders, and considered for the assessment of the safety issue. This is not a mandatory step of the procedure.
For detailed information on how and when to submit data please refer to Question 19 and Question 20.
Regardless of whether or not the MAHs present written or oral explanations to the Pharmacovigilance Risk Assessment Committee (PRAC), a recommendation applicable to all marketing authorisations concerned by the procedure will be issued by the PRAC.
The safety issue under urgent consideration by the Pharmacovigilance Risk Assessment Committee (PRAC) will be substantiated by additional data that could be requested in the format of a list(s) of questions, comments to the scientific background supporting the triggering of the procedure or by using data sources available to the Agency and/or to the national competent authorities (NCAs) of the Member States.
This data may be gathered from several different sources (i.e. from concerned marketing authorisation holders (MAHs), healthcare professionals, patients’ organisations, the public, Eudravigilance data, data available to the NCAs, etc.).
The need for specific data to be collected is identified by the PRAC at the start of the procedure and announced on the Agency’s website.
The data to be considered for the assessment will have to be submitted within the specified deadline as published in the Article 107i pharmacovigilance procedure page (please refer to Question 9). The time limit for submission of data should not exceed 20 days.
Notwithstanding the above, the PRAC may in some specific cases also collect additional data through a public hearing and/or an oral explanation (please refer to Question 24).
The assessment of data within an urgent Union procedure is led by the Pharmacovigilance Risk Assessment Committee (PRAC). At the start of the procedure, the PRAC Chairperson appoints a PRAC rapporteur and PRAC co-rapporteur(s) who will perform the assessment of all data collected within the agreed timelines.
The assessment will result in the PRAC issuing a recommendation on the safety issue reviewed, which will be forwarded to the Committee for Medicinal Products for Human Use (CHMP) or to the Co-ordination Group for Mutual Recognition and Decentralised Procedures (CMDh), as applicable (please refer to Question 32).
Even though the assessment of the urgent Union procedure will be performed by the PRAC, there will be a close collaboration during the assessment between the PRAC (co-)rapporteurs and the CHMP (co?)rapporteurs (in case at least one centrally authorised product is included in the scope of the procedure) or the CMDh member with the leading role (in case the concerned products are only nationally authorised including via the mutual recognition and decentralised procedures).
The Pharmacovigilance Risk Assessment Committee (PRAC) (co-)rapporteurs for an urgent Union procedure should be appointed by the PRAC Chairperson from amongst its members or alternates (hereafter referred to as PRAC members).
The PRAC Chairperson will endeavour to apply the criteria of best available expertise to be taken into account for the appointment of the PRAC (co-)rapporteurs for each urgent Union procedure.
In case of an urgent Union procedure concerning several active substances belonging to the same therapeutic class, or where several issues are to be assessed, a lead rapporteur and several co-rapporteurs may be appointed.
References:
During the assessment
The Article 107i pharmacovigilance procedure page will specify the modalities for submission of data relevant to the procedure (e.g. response to the Pharmacovigilance Risk Assessment Committee (PRAC) list of questions, comments to the scientific background supporting the triggering of the procedure).
Considering the urgency of the matter, all information available by stakeholders (e.g. marketing authorisation holders (MAHs), healthcare professionals, patients’ organisations, general public) must be provided by the date published in the announcement.
Marketing authorisation holder(s)
The MAHs of medicinal products concerned by the procedure should submit their responses as follows:
- The data should be presented in electronic format according to the electronic Common Technical Document (eCTD)/CTD format and accompanied by a signed cover letter and a written summary of each question.
- The cover letter must make clear reference to the procedure number and the Agency’s procedure manager who should always be put in copy.
- The written summary answering each question should follow the numbering as per the published PRAC list of questions. Please note that supportive data to the responses submitted (e.g. study reports, literature data, risk management plan) are expected to be provided together with a summary of those data as per the modular structure of the CTD format.
Published data can be presented as supportive documentation in response to a specific question if no other data is available.
In case some questions (e.g. on a specific pharmaceutical form) are not applicable/relevant to all medicinal product(s) concerned by the procedure or to the medicinal product(s) of the represented group, the response should be “not applicable” with a short explanation.
It should be noted that the responsibility for the quality of the submitted documentation lies with the MAHs and is crucial to the overall assessment of the safety issue. The data presented in the submissions should be intended exclusively for the purposes of the concerned procedure. The information and data contained in the individual submissions will be assessed and reflected in the assessment reports related to the concerned procedure. As a general rule, such information and data will not be redacted from the assessment reports with respect to individual products prior to sharing them with all concerned MAHs. Indeed for transparency reasons and in order to respect the right of defence of the MAHs concerned by the procedure, the Agency will share all the information and data relevant for the scientific assessment with all concerned MAHs. Moreover in general, it is not expected that individual submissions by the MAHs will include commercially confidential information.
It should be noted that neither the Agency nor the MAHs can use the information and data contained in the submissions for any other purposes than those related to the concerned procedure.
All submissions are expected to be submitted in English and electronically only (please refer to Question 20). Submission of responses concerning the Article 107i pharmacovigilance procedure with regards to centrally authorised products (CAPs) should follow the requirements for post-authorisation procedures for CAPs (e.g. submission via e-CTD).
In case MAHs formed a group (please refer to Question 12), the cover letter accompanying the single consolidated response and/or request for oral explanation should clearly identify the parties responsible for the submission/request.
Other stakeholders
All submissions from other stakeholders than the MAHs concerned by the procedure, (e.g. healthcare professionals, patients’ organisations, general public), have to be accompanied by a submission form with all its mandatory fields duly completed. This is a requirement for data to be considered for assessment. The submission form template is available on the urgent Union procedure page, under the tab dedicated to the submission of data by other stakeholders than MAHs.
The other requirement is the submission of all data within the deadline specified on the page.
In all cases, data submitted should be accompanied by an overall summary of its content and should make reference to the specific PRAC question being addressed (as per PRAC published list of questions numbering).
It is of the utmost importance that data is provided in due course to avoid undermining the safety review. In the same sense and considering the time constraints, data submission in English and electronically is strongly advised, as any requirements for translations would delay the assessment.
Marketing authorisation holder(s) (MAHs)
Responses from the MAHs should be submitted within the timeline specified on the procedure page. All submissions for referral procedures should be sent via the eSubmission Gateway or eSubmission Web Client. These portals send automated acknowledgement of receipt of submission, or of failed submission if an error occurred. Responses for nationally authorised products (NAPs) and for centrally authorised products (CAPs) submitted via these portals are available in the common repository and will be considered delivered to all Committee members and alternates.
The Agency no longer accepts electronic submissions for referrals on CD or DVD. Additional copies of submissions should not be sent directly to the NCAs on CD/DVD or via common European submission portal (CESP) as this might cause delays in processing the submissions.
For advanced therapy medicinal product (ATMP), additional submission requirements apply. Please refer to the dossier requirements for centrally authorised products.
The Agency strongly recommends using the electronic Common Technical Document (eCTD) or NeeS (Non-eCTD electronic Submission) formats for submissions related to referrals.
For referral submissions related to CAPs, it is mandatory to use the eCTD format. It is not possible to submit a standalone joint eCTD for the CAPs concerned. Responses should always be submitted individually as the next sequence in each CAP product lifecycle. However for submissions related to NAPs, if the documentation is common to several medicinal products and/or MAHs, a single joint eCTD should be submitted. Additional copies of the same eCTD should not be submitted.
For all types of submissions, responses should be presented in the modular format.
Recommended folder structure:
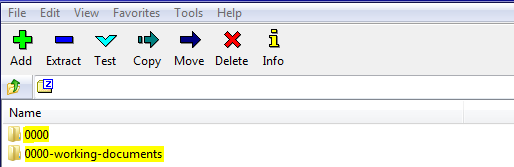
Documentation can be included in respective modules following the CTD location as referenced in the recommended folder structure, further, root folder should be 4 digits (between 0000-9999), e.g. Submission 0000 as below:

Any working documents (for example: documents in Word format) should be outside the root submission folder, e.g. as following:
Information on the required naming conventions and file formats can be found in detailed examples of filenames for different application types and in the eSubmission Gateway Web Client - Guidance for Applicants. For more information please refer to eSubmission website.
There is no need to send any separate paper cover letters for these submissions, as the cover letter will be in the relevant part of eCTD module 1 in PDF format.
Should you have any questions regarding your submission, please contact us via email:AskEMA, for any technical issues visit the EMA Service Desk portal.
Other stakeholders
Other stakeholders than the MAHs concerned by the procedure, (e.g. healthcare professionals, patients’ organisations, general public) can submit to the Agency data relevant to the assessment of the safety issue. Submission should be sent via email, to the address indicated on the procedure page, under the tab dedicated to the submission of data by stakeholders.
All submissions of responses from other stakeholders should be submitted within the timeline specified on the procedure page.
References:
Submissions from the marketing authorisation holders (MAHs) are directly available in the common repository to the Pharmacovigilance Risk Assessment Committee (PRAC) (co-)rapporteurs to be considered for the assessment.
The Agency will provide the data received from other stakeholders (e.g. healthcare professionals, patients’ organisations, general public) to the PRAC (co-)rapporteur to be considered for the assessment.
All information gathered will be assessed within the agreed timeframe as published on the Article 107i procedure page. The assessment report(s) prepared by the PRAC (co-)rapporteurs will reflect all data submitted and considered for the review.
A listing of the data received from other stakeholders will be annexed to the PRAC (co-)rapporteur’s assessment report(s) and also to the PRAC assessment report, which is published for transparency purposes and public awareness following the PRAC recommendation.
The PRAC (co-)rapporteur’s assessment report(s) will be circulated to the PRAC members for comments. These will also be shared with the Committee for Medicinal Products for Human Use (CHMP) (co-)rapporteurs (in case at least one centrally authorised product is included in the scope of the procedure) or with the Co-ordination Group for Mutual Recognition and Decentralised Procedures (CMDh) member with a leading role (in case the concerned products are only nationally authorised including via the mutual recognition and decentralised procedures) for comments, as applicable (please refer to Question 17).
Please note that the timelines provided below are for guidance purposes only.
The timelines following a 60 day assessment period are as follows:
| Article 107 Urgent Union Procedure – Timetable for the assessment | Day1 |
| Assessment starts on the next PRAC meeting2 following submission of all data (as per the published deadline) | Day 1
|
| PRAC (co)-rapporteurs circulate the assessment report(s) on the data collected | Day 20 |
| Comments in writing from PRAC members, CHMP concerned Rapporteur(s) (if at least one CAP is involved), CMD(h) member with leading role (if applicable) | Day 35 |
| PRAC (co-) rapporteurs circulate an updated assessment report(s) based on the comments received and reflecting any additional information received (if applicable) | Day 45 |
PRAC Recommendation
| Day 60 |
1The 60 days for assessment should be considered as the period of 3 consecutive PRAC plenary meetings.
2Corresponds to the 2nd PRAC meeting following the receipt of the notification triggering the procedure.
The dates to be followed in accordance with the above timetable for each month can be found in Procedural timetables
The PRAC) has a maximum of 60 days to issue a recommendation from the PRAC meeting after the deadline for the submission of all data as published at the time of announcement. However, in case of a justified urgency the PRAC may agree on a shorter timetable.
Additional procedural steps within the same timeframe (i.e. maximum of 60 days) may be necessary before the PRAC issues a recommendation. This applies in case of oral explanation(s) by the concerned marketing authorisation holder(s), public (and non-public) hearing or in case the PRAC requires input from a scientific advisory group or from an ad-hoc expert group to support the PRAC recommendation.
All marketing authorisation holder(s) (MAHs) with medicinal products included in the scope of the urgent Union procedure will be provided with the Pharmacovigilance Risk Assessment Committee (PRAC) (co-)rapporteur's assessment report(s) electronically via email/Eudralink.
As a general rule, other stakeholders will not be provided with the PRAC (co-)rapporteur's assessment report. In any case, the PRAC recommendation and assessment report will be published on the Agency website together with the final outcome of the Committee for Medicinal Products for Human Use (CHMP)/Co-ordination Group for Mutual Recognition and Decentralised Procedures (CMDh), as applicable (please refer to Question 30).
The Pharmacovigilance Risk Assessment Committee (PRAC), depending on the urgency of the matter, may decide that issues need to be addressed orally by the marketing authorisation holders (MAHs). In such a case, the MAHs will be duly informed in advance of the issues to be addressed during the oral explanation.
The MAHs may also request to the PRAC to present their views in an oral explanation. In such a case, the MAHs should send to the EMA procedure manager a request addressed to the PRAC stating the reason(s) and specifying the issue(s) to be addressed during the oral explanation. The PRAC will take due account of the request considering the urgency of the matter, and will decide whether the oral explanation will be held.
Oral explanation(s) should take place during the assessment phase and after the receipt of the PRAC (co-)rapporteur’s assessment report(s). Further detailed information on organisational aspects of the oral explanation can be found in
The MAHs can provide the oral explanation on their own behalf or on behalf of a group of MAHs.
Where the urgency of the matter permits, the PRAC may also hold public hearings, on justified grounds, particularly with regard to the extent and seriousness of the safety concern.
When the PRAC is of the opinion that a public hearing should be convened, the hearing shall be held in accordance with the modalities and rules specified by the Agency and will be announced on the Agency’s website. The announcement will also specify the modalities of participation. Further information can be found in Public hearings
In case the PRAC has decided to hold a public hearing, a MAH or another person intending to submit confidential data relevant to the subject matter of the procedure may request permission to present that data to the PRAC in a non-public hearing. Such request should be duly justified on the grounds of confidentiality of the data to be presented. A non-public hearing can only be held whenever the PRAC has decided to hold a public hearing.
If the marketing authorisation (MA) for a concerned product is transferred during the procedure, both the former and the new marketing authorisation holder (MAH) should update the Article 57 database without delay.
For a nationally authorised product (including via mutual recognition and decentralised procedures), the new MAH should provide a copy of the transfer decision of the relevant competent authority and, if relevant, information on the new contact person, to the EMA procedure manager (please refer to Question 11). Following receipt of the transfer decision, the Agency will inform the former MAH that they are no longer included in the urgent Union procedure, in relation to the MA transferred.
For a centrally authorised product (CAP), if during the procedure the MA is transferred, the former MAH should inform the EMA procedure manager and the appropriate procedure should be followed (please refer to Transfer of marketing authorisation: questions and answers).
The product listing published at the start of the procedure (please refer to Question 9) will be updated accordingly and republished on the Agency’s website on the specific procedure page.
If during the procedure, the name of a concerned product changes or, if the name and/or address of the marketing authorisation holder (MAH) changes or, if the marketing authorisation (MA) is withdrawn, the MAH should update the Article 57 database without delay and inform the EMA procedure manager.
For a nationally authorised product (including via mutual recognition and decentralised procedures), following official confirmation of the change, the Agency will inform the MAH/applicant that the change has been noted. If the Article 57 database is updated within 30 days of the start of the procedure, these changes will be included in the revised product listing that will be published at day 30. After day 30 the product listing will not be subject to any changes except in case of MA transfer (please refer to Question 25).If the contact person changes, the MAH should also inform the EMA procedure manager and, if the contact person is the QPPV, update the Article 57 database without delay.
For a centrally authorised product (CAP), if during the procedure, the name of the product or the name and/or address of the MAH changes or, if the contact person changes or, if the MA is withdrawn, the MAH should inform the EMA procedure manager. and the appropriate procedure should be followed (please refer to Changing the (invented) name of a centrally authorised medicine: questions and answers , Other post-authorisation activities: questions and answers and Withdrawn-product notification: questions and answers - MAH notification time ).
Pharmacovigilance Risk Assessment Committee (PRAC) recommendation
The Pharmacovigilance Risk Assessment Committee (PRAC) has a maximum of 60 days to issue a recommendation after the deadline for submission of all data as published at time of announcement (please refer to Question 22). The PRAC recommendation will usually be adopted on the last day of the PRAC's plenary meeting.
However, in case of justified urgency, the PRAC may issue a recommendation on a shorter timeframe and if needed by written procedure (i.e. outside a scheduled plenary meeting).
The Pharmacovigilance Risk Assessment Committee (PRAC) recommendation shall include any or a combination of the following conclusions:
- no further evaluation or action is required at Union level;
- the marketing authorisation holder (MAH) should conduct further evaluation of data together with the follow-up of the results of that evaluation;
- the MAH should sponsor a post-authorisation safety study (PASS) together with the follow up evaluation of the results of that study;
- risk minimisation measures should be implemented;
- the marketing authorisation (MA) should be suspended, revoked or not renewed;
- the MA should be varied.
With regards to point (d), the recommendation will specify the risk minimisation measures recommended and any conditions or restrictions to which the MA should be made subject.
With regards to point (f), where the recommendation is for the MA to be varied, including changes to the information in the summary of product characteristics (SmPC), labelling and/or package leaflet (PL), the recommendation will include the suggested wording of such amendments.
The PRAC recommendation can be adopted by consensus or by majority vote. In the event of adoption by majority, the divergent positions of the concerned PRAC members and the grounds on which they are based will be appended to the recommendation issued by the PRAC.
References:
The Pharmacovigilance Risk Assessment Committee (PRAC) recommendation will include:
a cover page in which the recommendation adopted is outlined together with the voting outcome of the PRAC;
a product listing including name of the medicinal products concerned/active substances and marketing authorisation holders (MAHs) for all identified products authorised nationally (including via the mutual recognition/decentralised procedures). In case centrally authorised products (CAPs) are involved their respective Annex A will be attached;
the scientific grounds and explanation for the PRAC recommendation;
the PRAC members’ divergent views, in case the recommendation is adopted by majority;
the PRAC assessment report on the evaluation performed and the conclusion of the PRAC that led to the adoption of the recommendation(s) based on all data gathered, including:
the wording (in English only) to be included in the relevant sections of the summary of product characteristics (SmPC), labelling and/or package leaflet (PL), if applicable;
the conditions or restrictions imposed on the marketing authorisation(s) for the safe and effective use of the medicinal product(s), if applicable;
the Direct Healthcare Professional Communication (DHPC) and communication plan as agreed by PRAC, if applicable.
The outcome of the Pharmacovigilance Risk Assessment Committee (PRAC) recommendation will be included in the PRAC meeting highlights that are released on the next working day following the PRAC plenary meeting together with a summary of the PRAC recommendation.
The PRAC assessment report detailing the PRAC recommendation will be published on the procedure page around one week following the adoption of the European Commission Decision or Co-ordination Group for Mutual Recognition and Decentralised Procedures (CMDh) consensus position as applicable (please refer to Question 40).
Reference:
The marketing authorisation holder(s) (MAHs) of medicinal product(s) concerned and identified at the start of the procedure will receive the Pharmacovigilance Risk Assessment Committee (PRAC) recommendation electronically via email/Eudralink during the week following the PRAC meeting when the recommendation was adopted.
The Pharmacovigilance Risk Assessment Committee (PRAC) recommendation on an urgent Union procedure is sent during the week following its adoption to:
the Committee for Medicinal Products for Human Use (CHMP), if at least one centrally authorised product is included in the procedure, for adoption of an opinion;
the Co-ordination Group for Mutual Recognition and Decentralised Procedures (CMDh), if only nationally authorised products (including via mutual recognition and decentralised procedures) are included in the procedure, to reach a position;
The CHMP or CMDh will consider the PRAC recommendation at their following plenary meeting and will agree on the timeframe needed to issue an opinion or position, respectively. This timeframe should not exceed 30 days after receipt of the PRAC recommendation (please refer to Question 33).
In parallel, some of the concerned marketing authorisation holders (MAHs) will be contacted by the Agency with a proposal for worksharing of the translation process in all EU official languages (please refer to Question 39).
Committee for Medicinal Products for Human Use (CHMP) opinion or Co-ordination Group for Mutual Recognition and Decentralised Procedures (CMDh) position/agreement
Following the receipt of the Pharmacovigilance Risk Assessment Committee (PRAC) recommendation, the Committee for Medicinal Products for Human Use (CHMP) or Co-ordination Group for Mutual Recognition and Decentralised Procedures (CMDh) will consider it at their plenary meeting. As a general rule, the aim will be to adopt the CHMP opinion or CMDh position in the month of the PRAC recommendation adoption.
However in some cases, the CHMP or CMDh may agree on the need to further consider the PRAC recommendation. In such cases, the CHMP opinion or CMDh position will be adopted within 30 days after receipt of the PRAC recommendation.
This decision will be reflected in the CHMP or CMDh meeting highlights published on the next working day following the plenary meetings.
The overall process including timelines for adoption of an opinion/position is the following:
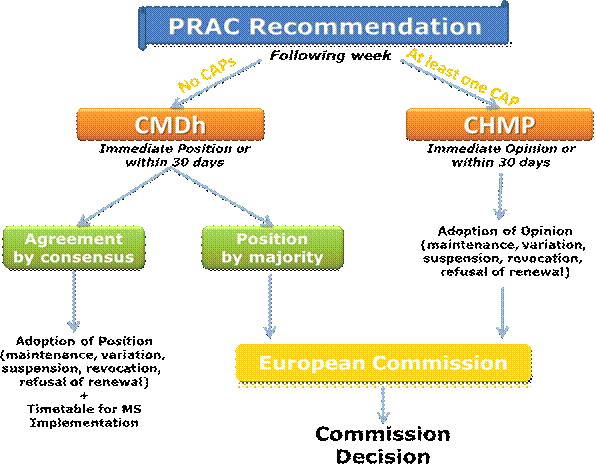
The Committee for Medicinal Products for Human Use (CHMP) or Co-ordination Group for Mutual Recognition and Decentralised Procedures (CMDh) will consider the Pharmacovigilance Risk Assessment Committee (PRAC) recommendation and assessment report and will adopt by consensus or by majority vote, an opinion or position respectively, on the maintenance, variation, suspension, revocation or non-renewal of the marketing authorisations (MAs) concerned (please refer to Question 28).
Exceptionally, an oral explanation may be held in front of the CHMP/CMDh. The CHMP/CMDh decides whether the oral explanation will be held.
Where the CHMP opinion or CMDh position differs from the recommendation of the PRAC, the CHMP or CMDh will attach to its opinion or position an explanation of the scientific grounds for the differences.
The Committee for Medicinal Products for Human Use (CHMP) opinion or Co-ordination Group for Mutual Recognition and Decentralised Procedures (CMDh) position will include:
a cover page in which the CHMP opinion or CMDh position adopted is outlined together with the voting outcome;
the Pharmacovigilance Risk Assessment Committee (PRAC) recommendation and its assessment report;
the scientific grounds and explanation for the opinion or position including a detailed explanation for any differences with the PRAC recommendation;
the CHMP/CMDh members’ divergent views, in case of adoption by majority instead of consensus;
the product listing including name of the medicinal products concerned/active substances and marketing authorisation holders (MAHs) for all identified products authorised nationally (including via the mutual recognition/decentralised procedures). In case centrally authorised products are involved their respective Annex A will be attached;
the wording (in English only) to be included in the relevant sections of the summary of product characteristics (SmPC), labelling and/or package leaflet (PL), if applicable (only for products authorised nationally including via the mutual recognition/decentralised procedures);
the revised product information with agreed wording included in the relevant sections of the SmPC, labelling and/or PL, if applicable (only for centrally authorised products);
the conditions or restrictions imposed on the marketing authorisation(s) for the safe and effective use of the medicinal product(s), if applicable;
the timetable for implementation of the CMDh position (only in case of consensus position);
the Direct Healthcare Professional Communication (DHPC) and communication plan as agreed by CHMP/CMDh, if applicable.
An EMA public health communication (including a summary of the CHMP opinion/CMDh position and targeted information for healthcare professional and patients) and, if applicable, the wording changes to be applied to the product information, and the timetable for implementation of the CMDh consensus position, will be published on the Friday of the plenary meeting week. In addition, a brief outcome of the Committee for Medicinal Products for Human Use (CHMP) opinion or Co-ordination Group for Mutual Recognition and Decentralised Procedures (CMDh) position, as applicable, will be included in the CHMP meeting highlights/CMDh report that are released following the plenary meetings.
The CHMP opinion or CMDh position will be published on the procedure page following the adoption of the European Commission Decision or CMDh consensus position as applicable (please refer to Question 40).
Reference:
The marketing authorisation holder(s) of medicinal products concerned and identified at the start of the procedure, will receive the Committee for Medicinal Products for Human Use (CHMP) opinion or Co-ordination Group for Mutual Recognition and Decentralised Procedures (CMDh) position electronically via email/Eudralink during the week following the CHMP or CMDh plenary meetings during which the CHMP opinion or CMDh position was adopted.
The marketing authorisation holders (MAHs) of medicinal products authorised nationally (including via the mutual recognition or decentralised procedures) will have to provide translations in all EU languages (including Icelandic and Norwegian, if applicable1) of the following annexes to the Committee for Medicinal Products for Human Use (CHMP) opinion or Co-ordination Group for Mutual Recognition and Decentralised Procedures (CMDh) position:
listing of nationally authorised products (including via mutual recognition/decentralised procedures) concerned by the procedure;
wording to be included in the relevant sections of the summary of product characteristics, labelling and/or package leaflet, if applicable.
Only one translation per EU language is required, therefore the MAHs actively involved in the procedure will be presented with a proposal for worksharing for the translation process. Not all MAHs may be involved in the translation process. MAHs that have not been contacted to participate in the worksharing process will be provided the set of translations at a later stage.
The Agency will contact the MAHs as early as possible to ensure the smooth running of the worksharing process. The translations will have to be provided to the Member States contact points for linguistic check by Day +5 (i.e. 5 days after adoption of the opinion or the position) and copied to the Agency. Member States may send linguistic comments until Day +19. The MAH should send the translations amended accordingly together with the completed Practical information on translations for referral procedures (human) to the Agency by Day +22.
The MAH(s) of centrally authorised products involved in the procedure will have to provide the full product information in all EU languages within the same timeframe (i.e. 5 days after adoption of the opinion) to the MSs contact points for linguistic check and copied to the Agency.
Detailed information on the translation process of the CHMP opinion or CMDh position can be found in Practical information on translations for referral procedures (human)
1 If authorised in Iceland and Norway
In case of a Committee for Medicinal Products for Human Use (CHMP) opinion or Co-ordination Group for Mutual Recognition and Decentralised Procedures (CMDh) position by majority vote, the Agency together with the concerned marketing authorisation holder(s) (MAHs) and national competent authorities (NCAs) in the Member States (MSs) will finalise the translations and send these to the European Commission (EC).
The EC will then start the decision-making process leading to the adoption of a binding decision addressed either to the MAHs or to MSs and notified to the MAHs, depending on whether the decision concerns centrally authorised products (CAPs) or nationally authorised products (including via the mutual recognition and decentralised procedures), respectively.
In case of CMDh position by consensus, the Agency together with the concerned MAHs and the NCAs in the MSs will finalise the translations. The consensus position will be implemented by the MSs in accordance with the timetable determined in the position (please refer to Question 33).
Detailed information on the decision-making process can be found here.
The MAHs of CAPs need to submit an eCTD closing sequence with the final documents within 5 days following the EC decision.
For NAPs, the CMDh recommendation for implementation of Commission Decisions or CMDh position can be found here.
The PRAC assessment report will be published on the procedure page, in English only, around one week following the adoption of the European Commission (EC) decision or Co-ordination Group for Mutual Recognition and Decentralised Procedures (CMDh) consensus position. Within four weeks of the adoption of the EC decision or eight weeks of the CMDh consensus position, the Committee for Medicinal Products for Human Use (CHMP) opinion or CMDh position (as applicable) with its annexes in all EU languages will be published on the procedure page. The page will also be updated to reflect the date of the EC decision or the CMDh consensus position, as applicable.
Reference: