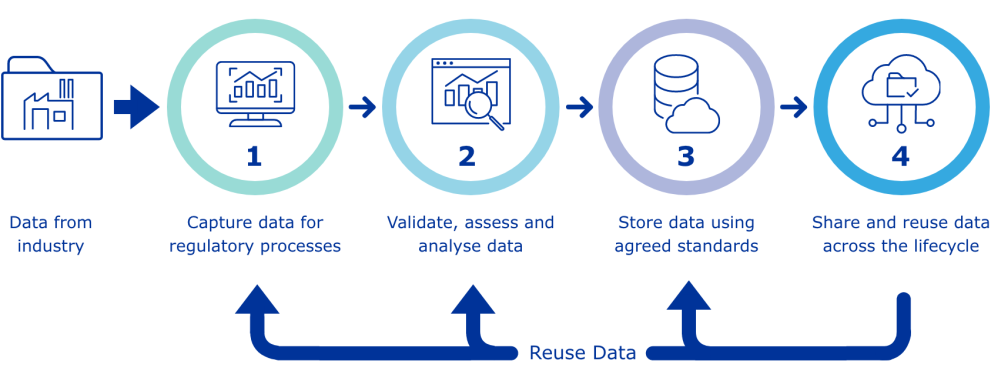
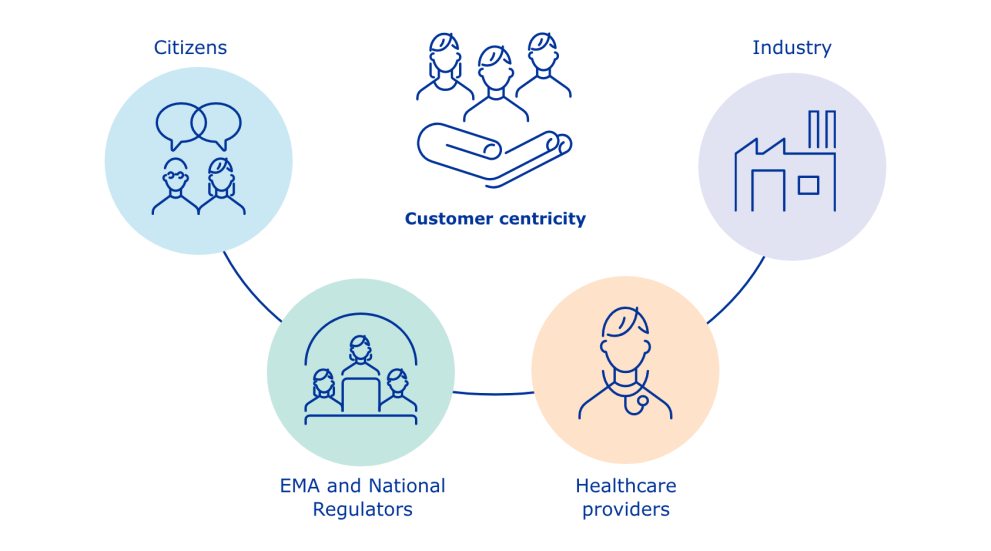
Substance and product data management services
The European Medicines Agency (EMA) is delivering a Product Management Service (PMS) and a Substance Management Service (SMS) to support regulatory activities in the European Union (EU).
Human
Data on medicines