Defect with Buccolam oral syringes
Press releaseHuman
A defect has been reported with Buccolam plastic syringes, and parents and carers should carefully inspect the syringes before giving the medicine to children.
Buccolam is an epilepsy medicine for children, which is available as pre-filled oral syringes. Parents or carers giving Buccolam should take the syringe out of the package, pull off the red cap and give the medicine in the side of the child's mouth when the child is having an epileptic seizure (fit).
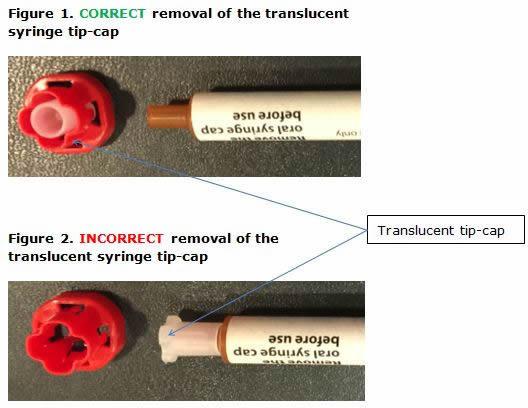
There have been a few cases where the red cap did not come off completely and a translucent tip-cap remained on the syringe, stopping the medicine from leaving the syringe. This has in some cases resulted in the tip-cap coming off inside the patient's mouth and being inhaled or ingested.
To make sure that the medicine is given correctly and safely, EMA is advising parents and carers to carefully check the syringe before giving Buccolam and to remove the translucent tip-cap if it is still on the syringe after the red cap has been pulled off.
A letter will be sent to all doctors and pharmacists who prescribe and dispense Buccolam, to alert them of this defect and encourage them to share this information with parents and carers of children who are using Buccolam. Practical advice and instructions will also be supplied to parents and carers who are, or have been, dispensed Buccolam.
The company that markets Buccolam is working to resolve this issue for new syringes. However, syringes already on the market in the EU and possibly already dispensed to patients need to be carefully checked before use.
Information for parents and carers
Information for healthcare professionals
More about the medicine
Buccolam is a medicine used to stop prolonged, acute (sudden) convulsive seizures (fits) in children and adolescents with epilepsy. Buccolam contains the active substance midazolam and is available as pre-filled syringes containing 2.5 mg, 5 mg, 7.5 mg or 10 mg of midazolam.