Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 11 to 14 September 2017
NewsHuman
Thirteen medicines recommended for approval, including one orphan medicine
The European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) recommended thirteen medicines for approval at its September meeting.
The CHMP recommended granting marketing authorisations for two cancer medicines: Zejula (niraparib), an orphan designated medicine intended for the treatment of ovarian cancer, and Tookad (padeliporfin), for the treatment of adenocarcinoma of the prostate.
Two medicines for the treatment of adults with moderate to severe chronic obstructive pulmonary disease also received a positive opinion from the Committee: Elebrato Ellipta (fluticasone furoate / umeclidinium / vilanterol) and Trelegy Ellipta (fluticasone furoate / umeclidinium / vilanterol).
CHMP gave positive opinions for two medicines for people using opioids: Nyxoid (naloxone), intended for the treatment of opioid overdose, and Zubsolv (buprenorphine / naloxone), intended for the treatment of opioid dependence. Hybrid applications were submitted for both medicines. This means that the marketing authorisation applications relied in part on the results of pre-clinical tests and clinical trials for a reference product and in part on new data.
The CHMP adopted a positive opinion for Tremfya (guselkumab) for the treatment of plaque psoriasis.
VeraSeal (human fibrinogen / human thrombin) received a positive opinion for use as a sealant during surgical operations in adults.
Two biosimilar medicines were recommended for approval by the Committee: Cyltezo (adalimumab) for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, axial spondyloarthritis, psoriatic arthritis, psoriasis, paediatric plaque psoriasis, hidradenitis suppurativa, Crohn's disease, ulcerative colitis and uveitis; and Ontruzant (trastuzumab) for the treatment of early and metastatic breast cancer, and metastatic gastric cancer. Ontruzant is the first trastuzumab biosimilar recommended for approval by the CHMP.
Three generic medicines received a positive opinion from the CHMP: Imatinib Teva B.V. (imatinib) for the treatment of leukaemia and gastrointestinal stromal tumours; Miglustat Gen.Orph (miglustat) for the treatment of mild to moderate type 1 Gaucher disease; and Ritonavir Mylan (ritonavir) for the treatment of HIV infection.
Outcome of re-examination of three negative recommendations
The applicants for Adlumiz (anamorelin hydrochloride), Human IgG1 monoclonal antibody specific for human interleukin-1 alpha XBiotech (human IgG1 monoclonal antibody specific for human interleukin-1 alpha) and Masipro (masitinib) requested re-examinations of the Committee's negative opinions for these medicines adopted at the May 2017 meeting. After considering the grounds for these requests, the CHMP re-examined the initial opinions and confirmed its previous recommendations to refuse the granting of marketing authorisations for these medicines.
For more information on these negative opinions, please see the questions-and-answers documents in the grid below.
Three recommendations on extensions of therapeutic indications
The Committee recommended extensions of indications for Tasigna, Firazyr and Stribild.
Negative opinion on one extension of therapeutic indication
The CHMP adopted a negative opinion for an extension of therapeutic indication for Raxone.
Outcome of review on factor VIII
The CHMP concluded that there is no clear and consistent evidence of a difference in the incidence of inhibitor development between the two classes of factor VIII medicines: those derived from plasma and those made by recombinant DNA technology. For more information please see the public health communication in the grid below.
Withdrawals of applications
Applications for initial marketing authorisations for Fulphila (pegfilgrastim), Ogivri (trastuzumab) and Tigecycline Accord (tigecycline) have been withdrawn.
Fulphila was intended to be used for reducing neutropenia in patients taking cancer treatments. Ogivri was intended to be used to treat breast and gastric cancers. Tigecycline Accord was intended to be used to treat certain complicated infections.
An application to extend the indication of Opdivo (nivolumab) to treat liver cancer has also been withdrawn.
Questions-and-answers documents on these withdrawals are available in the grid below.
Agenda and minutes
The agenda of the September 2017 meeting is published on EMA's website. Minutes of the July 2017 CHMP meeting will be published in the coming weeks.
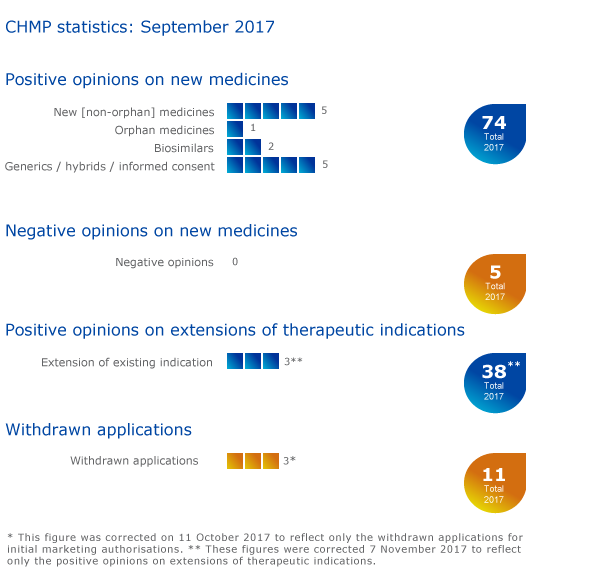
CHMP statistics
Key figures from the September 2017 CHMP meeting are presented in the graphic below.
More information on all other outcomes of the CHMP September 2017 meeting is available in the grid below.
CHMP statistics: September 2017
| Name of medicine | Elebrato Ellipta |
|---|---|
| International non-proprietary name (INN) | fluticasone furoate / umeclidinium / vilanterol |
| Marketing-authorisation applicant | GlaxoSmithKline Trading Services |
| Therapeutic indication | Treatment of adults with moderate to severe chronic obstructive pulmonary disease |
| More information | CHMP summary of positive opinion for Elebrato Ellipta |
| Name of medicine | Tremfya |
|---|---|
| INN | guselkumab |
| Marketing-authorisation applicant | Janssen-Cilag International N.V. |
| Therapeutic indication | Treatment of plaque psoriasis |
| More information | CHMP summary of positive opinion for Tremfya |
| Name of medicine | Nyxoid |
|---|---|
| INN | naloxone |
| Marketing-authorisation applicant | Mundipharma Corporation Limited |
| Therapeutic indication | Treatment of opioid overdose |
| More information | CHMP summary of positive opinion for Nyxoid |
| Name of medicine | Tookad |
|---|---|
| INN | padeliporfin |
| Marketing-authorisation applicant | STEBA Biotech S.A. |
| Therapeutic indication | Treatment of adenocarcinoma of the prostate |
| More information | CHMP summary of positive opinion for Tookad |
| Name of medicine | Trelegy Ellipta |
|---|---|
| INN | fluticasone furoate / umeclidinium / vilanterol |
| Marketing-authorisation applicant | GlaxoSmithKline Trading Services |
| Therapeutic indication | Treatment of adults with moderate to severe chronic obstructive pulmonary disease |
| More information | CHMP summary of positive opinion for Trelegy Ellipta |
| Name of medicine | VeraSeal |
|---|---|
| INN | human fibrinogen/human thrombin |
| Marketing-authorisation applicant | Instituto Grifols, S.A. |
| Therapeutic indication | Use as a sealant during surgical operations in adults |
| More information | CHMP summary of positive opinion for VeraSeal |
| Name of medicine | Zejula |
|---|---|
| INN | niraparib |
| Marketing-authorisation applicant | Tesaro UK Limited |
| Therapeutic indication | Treatment of ovarian cancer |
| More information | CHMP summary of positive opinion for Zejula |
| Name of medicine | Zubsolv |
|---|---|
| INN | buprenorphine / naloxone |
| Marketing-authorisation applicant | Mundipharma Corporation Limited |
| Therapeutic indication | Treatment of opioid dependence |
| More information | CHMP summary of positive opinion for Zubsolv |
| Name of medicine | Imatinib Teva B.V. |
|---|---|
| INN | imatinib |
| Marketing-authorisation applicant | Teva B.V. |
| Therapeutic indication | Treatment of leukaemia and gastrointestinal stromal tumours |
| More information |
| Name of medicine | Miglustat Gen.Orph |
|---|---|
| INN | miglustat |
| Marketing-authorisation applicant | Gen.Orph |
| Therapeutic indication | Treatment of mild to moderate type 1 Gaucher disease |
| More information | CHMP summary of positive opinion for Miglustat Gen.Orph |
| Name of medicine | Ritonavir Mylan |
|---|---|
| INN | ritonavir |
| Marketing-authorisation applicant | MYLAN S.A.S |
| Therapeutic indication | Treatment of HIV infection |
| More information | CHMP summary of positive opinion for Ritonavir Mylan |
| Name of medicine | Cyltezo |
|---|---|
| INN | adalimumab |
| Marketing-authorisation applicant | Boehringer Ingelheim International GmbH |
| Therapeutic indication | Treatment of rheumatoid arthritis, juvenile idiopathic arthritis, axial spondyloarthritis, psoriatic arthritis, psoriasis, paediatric plaque psoriasis, hidradenitis suppurativa, Crohn's disease, ulcerative colitis and uveitis |
| More information |
| Name of medicine | Ontruzant |
|---|---|
| INN | trastuzumab |
| Marketing-authorisation applicant | Samsung Bioepis UK Limited (SBUK) |
| Therapeutic indication | Treatment of early and metastatic breast cancer, and metastatic gastric cancer |
| More information | CHMP summary of positive opinion for Ontruzant |
| Name of medicine | Adlumiz |
|---|---|
| INN | anamorelin hydrochloride |
| Marketing-authorisation applicant | Helsinn Birex Pharmaceuticals Ltd |
| Therapeutic indication | Treatment of anorexia, cachexia or unintended weight loss in adult patients with non-small cell lung cancer |
| More information | Questions and answers on refusal of the marketing authorisation for Adlumiz (anamorelin hydrochloride) |
| Name of medicine | Human IgG1 monoclonal antibody specific for human interleukin-1 alpha XBiotech |
|---|---|
| INN | human IgG1 monoclonal antibody specific for human interleukin-1 alpha |
| Marketing-authorisation applicant | XBiotech Germany GmbH |
| Therapeutic indication | Treatment of debilitating symptoms of advanced colorectal cancer |
| More information | Questions and answers on refusal of the marketing authorisation for Human IgG1 monoclonal antibody specific for human interleukin-1 alpha XBiotech |
| Name of medicine | Masipro |
|---|---|
| INN | masitinib |
| Marketing-authorisation applicant | AB Science |
| Therapeutic indication | Treatment of systemic mastocytosis |
| More information | Questions and answers on refusal of the marketing authorisation for Masipro (masitinib) |
| Name of medicine | Firazyr |
|---|---|
| INN | icatibant |
| Marketing-authorisation holder | Shire Orphan Therapies GmbH |
| More information | CHMP post-authorisation summary of positive opinion for Firazyr (II-34-G) |
| Name of medicine | Stribild |
|---|---|
| INN | elvitegravir / cobicistat / emtricitabine / tenofovir disoproxil |
| Marketing-authorisation holder | Gilead Sciences International Limited |
| More information | CHMP post-authorisation summary of positive opinion for Stribild |
| Name of medicine | Tasigna |
|---|---|
| INN | nilotinib |
| Marketing-authorisation holder | Novartis Europharm Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Tasigna |
| Name of medicine | Raxone |
|---|---|
| INN | idebenone |
| Marketing-authorisation holder | Santhera Pharmaceuticals (Deutschland) GmbH |
| More information | Questions and answers on the refusal of a change to the marketing authorisation for Raxone (idebenone) |
| Name of medicine | Factor VIII |
|---|---|
| More information | Factor VIII medicines: no clear and consistent evidence of difference in risk of inhibitor development between classes |
| Name of medicine | Fulphila |
|---|---|
| INN | pegfilgrastim |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Fulphila (pegfilgrastim) |
| Name of medicine | Ogivri |
|---|---|
| INN | trastuzumab |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Ogivri (trastuzumab) |
| Name of medicine | Tigecycline Accord |
|---|---|
| INN | tigecycline |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Tigecycline Accord (tigecycline) |
| Name of medicine | Opdivo |
|---|---|
| INN | nivolumab |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Opdivo (nivolumab) |
| Name of medicine | Benlysta |
|---|---|
| INN | belimumab |
| Marketing-authorisation holder | Glaxo Group Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Benlysta (X-46-G) |