Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 15-18 December 2014
NewsHuman
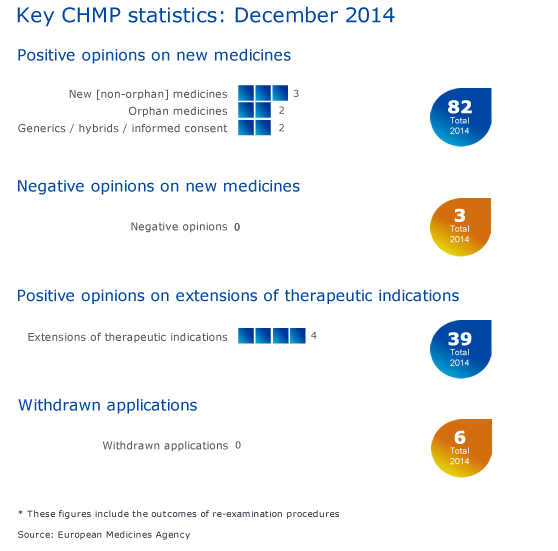
Seven new medicines recommended for approval in December 2014, 82 overall in 2014
Seven new medicines have been recommended for marketing authorisation at the December 2014 meeting of the Committee for Medicinal Products for Human Use (CHMP). This brings the total number of medicines recommended for approval by the CHMP in 2014 to 82.
The CHMP has recommended granting a conditional marketing authorisation for the orphan medicine Holoclar for the treatment of moderate to severe limbal stem cell deficiency due to physical or chemical burns to the eyes in adults. Holoclar is the first advanced therapy medicine containing stem cells to be recommended for approval in the European Union. For more information please see the press release in the grid below.
The Committee also gave a positive opinion for Mysimba (naltrexone / bupropion) for weight management in adults who are obese, or those who are overweight and have one or more complications related to their weight. The medicine is recommended for use in addition to a reduced-calorie diet and physical activity. For more information please see the press release in the grid below.
Xadago (safinamide) received a positive opinion for the treatment of Parkinson's disease.
Xydalba (dalbavancin) received a positive opinion for the treatment of skin and skin structure infections. The orphan medicine Quinsair (levofloxacin) has been recommended for the treatment of chronic pulmonary infections due to Pseudomonas aeruginosa in adult patients with cystic fibrosis.
The CHMP granted a positive opinion for the informed consent application Tasermity (sevelamer hydrochloride) for the control of hyperphosphataemia in adults receiving dialysis. In an informed consent application, reference is made to an authorised medicine and the marketing authorisation holder of the reference medicine has given consent to the use of their dossier in the application procedure.
The generic medicine Clopidogrel ratiopharm (clopidogrel) received a positive opinion for the prevention of myocardial infarction, ischaemic stroke, peripheral arterial disease and acute coronary syndrome, as well as the prevention of atherothrombotic and thromboembolic events in atrial fibrillation.
Four recommendations on extensions of therapeutic indications
The Committee recommended extensions of indications for four medicines: Revlimid, Tresiba, Velcade and Xiapex.
Update on GVK Biosciences review
The review procedure for GVK Biosciences is ongoing. In line with the published timetable, the CHMP is expected to give a recommendation on this issue following its January 2015 meeting.
Agenda and minutes
The agenda of the December 2014 meeting is published on the EMA website. The minutes of the December meeting will be published during the week following the January 2015 CHMP meeting.
CHMP statistics
Key figures from the December 2014 CHMP meeting and overall figures for 2014 are represented in the graphic below.
| Name of medicine | Holoclar |
|---|---|
| Common name | (ex-vivo) autologous corneal epithelial cells including stem cells |
| Marketing-authorisation applicant | Chiesi Farmaceutici S.p.A. |
| Therapeutic indication | Treatment of limbal stem cell deficiency |
| More information |
CHMP summary of positive opinion for Holoclar Press release: First stem-cell therapy recommended for approval in EU |
| Name of medicine | Mysimba |
|---|---|
| International non-proprietary name (INN) | naltrexone / bupropion |
| Marketing-authorisation applicant | Orexigen Therapeutics Ireland Limited |
| Therapeutic indication | Management of obesity |
| More information |
CHMP summary of positive opinion for Mysimba Press release: Mysimba recommended for approval in weight management in adults |
| Name of medicine | Quinsair |
|---|---|
| INN | levofloxacin |
| Marketing-authorisation applicant | Aptalis Pharma SAS |
| Therapeutic indication | Treatment of chronic pulmonary infections due to Pseudomonas aeruginosa in adult patients with cystic fibrosis |
| More information | CHMP summary of positive opinion for Quinsair |
| Name of medicine | Xadago |
|---|---|
| INN | safinamide |
| Marketing-authorisation applicant | Zambon SpA |
| Therapeutic indication | Treatment of Parkinson's disease |
| More information | CHMP summary of positive opinion for Xadago |
| Name of medicine | Xydalba |
|---|---|
| INN | dalbavancin |
| Marketing-authorisation applicant | Durata Therapeutics International B.V. |
| Therapeutic indication | Treatment of skin and skin structure infections |
| More information | CHMP summary of positive opinion for Xydalba |
| Name of medicine | Tasermity |
|---|---|
| INN | sevelamer hydrochloride |
| Marketing-authorisation applicant | Genzyme Europe BV |
| Therapeutic indication | Control of hyperphosphataemia |
| More information | CHMP summary of positive opinion for Tasermity |
| Name of medicine | Clopidogrel ratiopharm |
|---|---|
| INN | clopidogrel |
| Marketing-authorisation applicant | Teva Pharma B.V. |
| Therapeutic indication |
Prevention of myocardial infarction, ischaemic stroke, peripheral arterial disease, acute coronary syndrome; Prevention of atherothrombotic and thromboembolic events in atrial fibrillation |
| More information | CHMP summary of positive opinion for Clopidogrel ratiopharm |
| Name of medicine | Revlimid |
|---|---|
| INN | lenalidomide |
| Marketing-authorisation holder | Celgene Europe Limited |
| More information | CHMP post-authorisation summary of positive opinion for Revlimid |
| Name of medicine | Tresiba |
|---|---|
| INN | insulin degludec |
| Marketing-authorisation holder | Novo Nordisk A/S |
| More information | CHMP post-authorisation summary of positive opinion for Tresiba |
| Name of medicine | Velcade |
|---|---|
| INN | bortezomib |
| Marketing-authorisation holder | Janssen-Cilag International N.V. |
| More information | CHMP post-authorisation summary of positive opinion for Velcade |
| Name of medicine | Xiapex |
|---|---|
| INN | collagenase clostridium histolyticum |
| Marketing-authorisation holder | Swedish Orphan Biovitrum AB |
| More information | CHMP post-authorisation summary of positive opinion for Xiapex |