Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 15-18 May 2017
NewsHuman
Eleven medicines recommended for approval, including one advanced therapy medicine
The European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) recommended eleven medicines, including one advanced therapy medicinal product (ATMP), for approval at its May 2017 meeting.
The CHMP recommended granting a marketing authorisation for the ATMP Spherox (spheroids of human autologous matrix-associated chondrocytes) to treat adult patients who have symptomatic articular cartilage defects in the knee where the size of the affected area is no larger than 10 cm². For more information, please see the press release in the grid below.
The CHMP recommended granting a marketing authorisation for Oxervate (cenegermin) for the treatment of moderate to severe neurotrophic keratitis. Oxervate has an orphan designation. For more information on this medicine, please see the press release in the grid below.
Reagila (cariprazine) received a positive opinion from the Committee for the treatment of schizophrenia.
The CHMP granted a positive opinion for Kyntheum (brodalumab) for the treatment of moderate to severe plaque psoriasis.
Trimbow (beclometasone / formoterol / glycopyrronium bromide) received a positive opinion for the treatment of moderate to severe chronic obstructive pulmonary disease (COPD).
Veltassa (patiromer) received a positive opinion for the treatment of hyperkalaemia.
Four biosimilar medicines were recommended for approval by the Committee: Insulin lispro Sanofi (insulin lispro) received a positive opinion for the treatment of diabetes mellitus. Three medicines with rituximab as their active substance received a positive opinion: Blitzima and Tuxella, for the treatment of non-Hodgkin's lymphoma, chronic lymphocytic leukaemia, granulomatosis with polyangiitis and microscopic polyangiitis; and Ritemvia, for the treatment of non-Hodgkin's lymphoma, granulomatosis with polyangiitis and microscopic polyangiitis.
A biosimilar medicine is a biological medicine that is highly similar to another biological medicine already authorised for use.
One generic medicine received a positive opinion from the CHMP: Efavirenz / Emtricitabine / Tenofovir disoproxil Zentiva (efavirenz / emtricitabine / tenofovir disoproxil) for the treatment of HIV infection.
Negative opinions on three new medicines
The CHMP adopted a negative opinion for Adlumiz (anamorelin hydrochloride). Adlumiz was expected to be used to treat anorexia, cachexia or unintended weight loss in patients with non-small cell lung cancer.
The Committee adopted a negative opinion for Human IgG1 monoclonal antibody specific for human interleukin-1 alpha XBiotech. This medicine was intended to treat debilitating symptoms of advanced colorectal cancer.
The Committee also adopted a negative opinion for Masipro (masitinib). Masipro was intended to be used to treat systemic mastocytosis.
For more information on these negative opinions, please see the questions-and-answers documents in the grid below.
Six recommendations on extensions of therapeutic indications
The Committee recommended extensions of indications for Izba, Komboglyze, Onglyza, Renvela, Sevelamer carbonate Zentiva and Zykadia.
Withdrawal of application
An application for an initial marketing authorisation for Qinprezo (vosaroxin) has been withdrawn. This medicine was intended to be used in combination with the cancer medicine cytarabine for the treatment of acute myeloid leukaemia. A questions-and-answers document on this withdrawal is available in the grid below.
Outcome of review on vancomycin antibiotics
The CHMP has recommended changes to the prescribing information for the antibiotic vancomycin to ensure appropriate use in the treatment of serious infections caused by Gram-positive bacteria. For more information please see the public health communication in the grid below.
Agenda and minutes
The agenda of the May 2017 meeting is published on EMA's website. Minutes of the April 2017 CHMP meeting will be published next week.
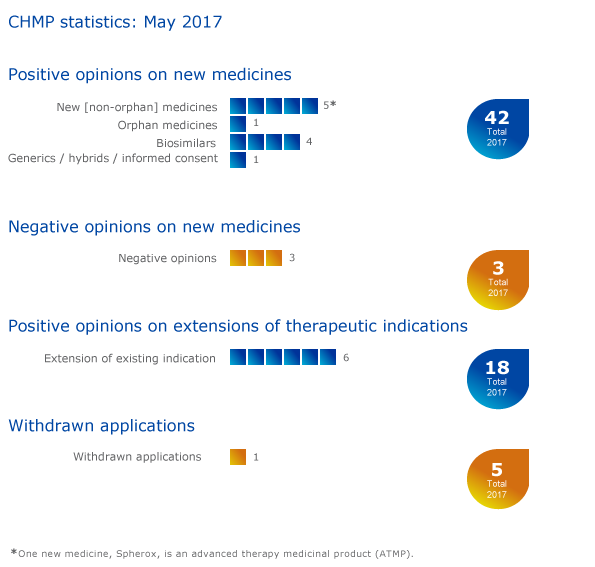
CHMP statistics
Key figures from the May 2017 CHMP meeting are represented in the graphic below.
More information on all other outcomes of the CHMP's May 2017 meeting is available in the grid below.
| Name of medicine | Kyntheum |
|---|---|
| INN | brodalumab |
| Marketing-authorisation applicant | LEO Pharma A/S |
| Therapeutic indication | Treatment of moderate to severe plaque psoriasis |
| More information | CHMP summary of positive opinion for Kyntheum |
| Name of medicine | Oxervate |
|---|---|
| INN | cenegermin |
| Marketing-authorisation applicant | Dompe farmaceutici s.p.a. |
| Therapeutic indication | Treatment of moderate to severe neurotrophic keratitis |
| More information |
Press release: New medicine for rare eye disease |
| Name of medicine | Reagila |
|---|---|
| INN | cariprazine |
| Marketing-authorisation applicant | Gedeon Richter |
| Therapeutic indication | Treatment of schizophrenia |
| More information | CHMP summary of positive opinion for Reagila |
| Name of medicine | Spherox |
|---|---|
| INN | spheroids of human autologous matrix-associated chondrocytes |
| Marketing-authorisation applicant | CO.DON AG |
| Therapeutic indication | Repair of certain cartilage defects of the knee |
| More information |
Press release: New advanced therapy to repair cartilage defects in the knee |
| Name of medicine | Trimbow |
|---|---|
| INN | beclometasone / formoterol / glycopyrronium bromide |
| Marketing-authorisation applicant | Chiesi Farmaceutici S.p.A. |
| Therapeutic indication | Treatment of moderate to severe chronic obstructive pulmonary disease |
| More information | CHMP summary of positive opinion for Trimbow |
| Name of medicine | Veltassa |
|---|---|
| INN | patiromer |
| Marketing-authorisation applicant | Vifor Fresenius Medical Care Renal Pharma France |
| Therapeutic indication | Treatment of hyperkalaemia |
| More information | CHMP summary of positive opinion for Veltassa |
| Name of medicine | Efavirenz / Emtricitabine / Tenofovir disoproxil Zentiva |
|---|---|
| INN | efavirenz / emtricitabine / tenofovir disoproxil |
| Marketing-authorisation applicant | Zentiva k.s. |
| Therapeutic indication | Treatment of HIV infection |
| More information | CHMP summary of positive opinion for Efavirenz/Emtricitabine/Tenofovir disoproxil Zentiva |
| Name of medicine | Blitzima |
|---|---|
| INN | rituximab |
| Marketing-authorisation applicant | Celltrion Healthcare Hungary Kft. |
| Therapeutic indication | Treatment of non-Hodgkin's lymphoma (NHL), chronic lymphocytic leukaemia, granulomatosis with polyangiitis and microscopic polyangiitis |
| More information | CHMP summary of positive opinion for Blitzima |
| Name of medicine | Insulin lispro Sanofi |
|---|---|
| International non-proprietary name (INN) | insulin lispro |
| Marketing-authorisation applicant | sanofi-aventis groupe |
| Therapeutic indication | Treatment of diabetes mellitus |
| More information | CHMP summary of positive opinion for Insulin lispro Sanofi |
| Name of medicine | Ritemvia |
|---|---|
| INN | rituximab |
| Marketing-authorisation applicant | Celltrion Healthcare Hungary Kft. |
| Therapeutic indication | Treatment of Non-Hodgkin's lymphoma, granulomatosis with polyangiitis and microscopic polyangiitis |
| More information | CHMP summary of positive opinion for Ritemvia |
| Name of medicine | Tuxella |
|---|---|
| INN | rituximab |
| Marketing-authorisation applicant | Celltrion Healthcare Hungary Kft. |
| Therapeutic indication | Treatment of non-Hodgkin's lymphoma, chronic lymphocytic leukaemia, granulomatosis with polyangiitis and microscopic polyangiitis |
| More information |
| Name of medicine | Adlumiz |
|---|---|
| INN | anamorelin hydrochloride |
| Marketing-authorisation applicant | Helsinn Birex Pharmaceuticals Ltd |
| Therapeutic indication | Treatment of anorexia, cachexia or unintended weight loss in patients with non-small cell lung cancer |
| More information | Questions and answers on refusal of the marketing authorisation for Adlumiz (anamorelin hydrochloride) |
| Name of medicine | Human IgG1 monoclonal antibody specific for human interleukin-1 alpha XBiotech |
|---|---|
| INN | human IgG1 monoclonal antibody specific for human interleukin-1 alpha |
| Marketing-authorisation applicant | XBiotech Germany GmbH |
| Therapeutic indication | Treatment of advanced colorectal cancer |
| More information | Questions and answers on refusal of the marketing authorisation for Human IgG1 monoclonal antibody specific for human interleukin-1 alpha XBiotech |
| Name of medicine | Masipro |
|---|---|
| INN | masitinib |
| Marketing-authorisation applicant | AB Science |
| Therapeutic indication | Treatment of systemic mastocytosis |
| More information | Questions and answers on refusal of the marketing authorisation for Masipro (masitinib) |
| Name of medicine | Izba |
|---|---|
| INN | travoprost |
| Marketing-authorisation holder | Novartis Europharm Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Izba |
| Name of medicine | Komboglyze |
|---|---|
| INN | saxagliptin / metformin hydrochloride |
| Marketing-authorisation holder | AstraZeneca AB |
| More information | CHMP post-authorisation summary of positive opinion for Komboglyze |
| Name of medicine | Onglyza |
|---|---|
| INN | saxagliptin |
| Marketing-authorisation holder | AstraZeneca AB |
| More information | CHMP post-authorisation summary of positive opinion for Onglyza (WS/1078) |
| Name of medicine | Renvela |
|---|---|
| INN | sevelamer carbonate |
| Marketing-authorisation holder | Genzyme Europe BV |
| More information | CHMP post-authorisation summary of positive opinion for Renvela (WS/965) |
| Name of medicine | Sevelamer carbonate Zentiva |
|---|---|
| INN | sevelamer carbonate |
| Marketing-authorisation holder | Genzyme Europe BV |
| More information | CHMP post-authorisation summary of positive opinion for Sevelamer carbonate Zentiva (WS/965) |
| Name of medicine | Zykadia |
|---|---|
| INN | ceritinib |
| Marketing-authorisation holder | Novartis Europharm Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Zykadia (II-12) |
| Name of medicine | Uptravi |
|---|---|
| INN | selexipag |
| Marketing-authorisation holder | Actelion Registration Ltd |
| More information |
| Name of medicine | Isentress |
|---|---|
| INN | raltegravir |
| Marketing-authorisation holder | Merck Sharp & Dohme Ltd |
| More information | CHMP summary of positive opinion for Isentress (X-59) |
| Name of medicine | Vancomycin containing medicines |
|---|---|
| INN | vancomycin |
| More information | EMA recommends changes to prescribing information for vancomycin antibiotics |
| Name of medicine | Cardioxane |
|---|---|
| INN | dexrazoxane |
| Marketing-authorisation holder | Clinigen Group |
| More information | Questions and answers on Cardioxane (dexrazoxane, powder for solution for injection, 500 mg) |
| Name of medicine | Paracetamol / Ibuprofen 500 mg / 150 mg film coated tablets and associated names |
|---|---|
| INN | Paracetamol / Ibuprofen 500 mg / 150 mg |
| Marketing-authorisation holder | Vale Pharmaceutical Ltd |
| More information | Questions and answers on Paracetamol / Ibuprofen 500 mg / 150 mg film coated tablets and associated names |
| Name of medicine | Qinprezo |
|---|---|
| INN | vosaroxin |
| More information | Questions and answers on the withdrawal of the application for the marketing authorisation for Qinprezo (vosaroxin) |