Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 17-20 February 2014
NewsHuman
This page provides an overview of the opinions adopted at the February 2014 meeting of the Committee for Medicinal Products for Human Use (CHMP) and other important outcomes.
Ten new medicines recommended for approval
The CHMP has recommended granting a paediatric-use marketing authorisation (PUMA) for Hemangiol (propranolol) for the treatment of proliferating infantile haemangioma. This is the second time that the Committee has granted a positive opinion for a PUMA since the introduction of this type of marketing authorisation by the Paediatric Regulation, which came into force in 2007. Please see the press release in the grid below for more information.
This month, a number of medicines for the treatment of chronic obstructive pulmonary disease (COPD) were recommended for marketing authorisation by the CHMP.
The Committee recommended granting marketing authorisations for six medicines for the treatment of respiratory diseases. Four of these, Anoro (umeclidinium bromide / vilanterol) and Laventair (umeclidinium bromide / vilanterol), as well as Incruse (umeclidinium bromide) and Ulunar Breezhaler (indacaterol / glycopyrronium bromide), are intended for the treatment of symptoms in adult patients with COPD. The remaining two, DuoResp Spiromax (budesonide / formoterol) and BiResp Spiromax (budesonide / formoterol), are intended for the treatment of asthma and COPD.
In other therapeutic areas, Vimizim (elosulfase alfa) was granted marketing authorisation by the CHMP for the treatment of mucopolysaccharidosis type IVA. Vimizim has an orphan designation.
The Committee recommended the granting of a marketing authorisation for Vokanamet (canagliflozin / metformin) for the treatment of type 2 diabetes.
Pregabalin Pfizer (pregabalin) was also recommended for approval by the Committee for the treatment of neuropathic pain, epilepsy and general anxiety disorder.
New compassionate-use programme
The CHMP has given an opinion on the use of a fixed-dose combination of ledipasvir and sofosbuvir for the treatment of chronic (long-term) hepatitis C virus (HCV) infection, in a compassionate-use programme. Please see the press release in the grid below for more information.
Outcome of two safety reviews
The CHMP has recommended restricting the use of methysergide-containing medicines due to concerns that it could cause fibrosis, a condition in which fibrous (scar) tissue accumulates in the body's organs potentially damaging them.
The CHMP concluded its review of Protelos/Osseor at its February meeting and has recommended further restricting the use of the medicine to patients who cannot be treated with other medicines approved for osteoporosis. For more information, please see the public health communication below.
Four requests for re-examination of CHMP recommendations
The marketing authorisation holders for Masiviera, Nerventra, Reasanz and Translarna have requested re-examinations of the CHMP's negative opinions for these medicines adopted during the January 2014 meeting. Upon receipt of the grounds of the requests for re-examination, the CHMP will re-examine these opinions and issue final opinions.
Withdrawal of application
A questions-and-answers document on the withdrawal of marketing authorisation application for Heplisav is also published below.
More information on these and other outcomes of this month's meeting are available in the table below.
CHMP welcomes new member
At the February 2014 CHMP, Dimitrios Kouvelas replaced Aikaterini Moraiti as the new CHMP member for Greece.
Agenda and minutes
The agenda of the February 2014 meeting is published on the EMA website. The minutes of the meeting will be published during the week following the March CHMP meeting. Minutes of the January 2014 CHMP meeting will be published next week.
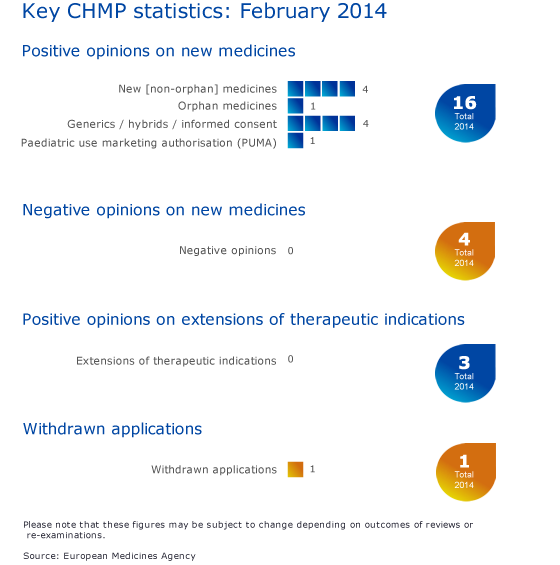
CHMP statistics
Key figures from the February 2014 CHMP meeting are represented in the graphic below.
| Name of medicine | Anoro |
|---|---|
| International non-proprietary name (INN) | umeclidinium bromide / vilanterol |
| Marketing-authorisation applicant | Glaxo Group Ltd |
| Therapeutic indication | Treatment of symptoms in adult patients with chronic obstructive pulmonary disease |
| More information | CHMP summary of positive opinion for Anoro |
| Name of medicine | Hemangiol |
|---|---|
| INN | propranolol |
| Marketing-authorisation applicant | Pierre Fabre Dermatologie |
| Therapeutic indication | Treatment of proliferating infantile haemangioma |
| More information | CHMP summary of positive opinion for Hemangiol |
| Press release: European Medicines Agency gives second positive opinion for a paediatric-use marketing authorisation |
| Name of medicine | Incruse |
|---|---|
| INN | umeclidinium bromide |
| Marketing-authorisation applicant | Glaxo Group Ltd |
| Therapeutic indication | Treatment of symptoms in adult patients with chronic obstructive pulmonary disease |
| More information | CHMP summary of positive opinion for Incruse |
| Name of medicine | Laventair |
|---|---|
| INN | umeclidinium bromide / vilanterol |
| Marketing-authorisation applicant | Glaxo Group Ltd |
| Therapeutic indication | Treatment of symptoms in adult patients with chronic obstructive pulmonary disease |
| More information | CHMP summary of positive opinion for Laventair |
| Name of medicine | Vimizim |
|---|---|
| INN | elosulfase alfa |
| Marketing-authorisation applicant | BioMarin Europe Ltd |
| Therapeutic indication | Treatment of mucopolysaccharidosis, type IVA |
| More information | CHMP summary of positive opinion for Vimizim |
| Name of medicine | Vokanamet |
|---|---|
| INN | canagliflozin / metformin |
| Marketing-authorisation applicant | Janssen-Cilag International N.V. |
| Therapeutic indication | Treatment of type 2 diabetes mellitus |
| More information | CHMP summary of positive opinion for Vokanamet |
| Name of medicine | Pregabalin Pfizer |
|---|---|
| INN | pregabalin |
| Marketing-authorisation applicant | Pfizer Limited |
| Therapeutic indication | Treatment of of neuropathic pain, epilepsy and generalised anxiety disorder |
| More information | CHMP summary of positive opinion for Pregabalin Pfizer |
| Name of medicine | Ulunar Breezhaler |
|---|---|
| INN | indacaterol / glycopyrronium bromide |
| Marketing-authorisation applicant | Novartis Europharm Ltd |
| Therapeutic indication | Treatment of chronic obstructive pulmonary disease |
| More information | CHMP summary of positive opinion for Ulunar Breezhaler |
| Name of medicine | BiResp Spiromax |
|---|---|
| INN | budesonide / formoterol |
| Marketing-authorisation applicant | Teva Pharma B.V. |
| Therapeutic indication | Treatment of asthma and chronic obstructive pulmonary disease |
| More information | CHMP summary of positive opinion for BiResp Spiromax |
| Name of medicine | DuoResp Spiromax |
|---|---|
| INN | budesonide / formoterol |
| Marketing-authorisation applicant | Teva Pharma B.V. |
| Therapeutic indication | Treatment of asthma and chronic obstructive pulmonary disease |
| More information | CHMP summary of positive opinion for DuoResp Spiromax |
| Name of medicine | Sofosbuvir / Ledipasvir |
|---|---|
| INN | sofosbuvir / ledipasvir |
| Applicant | Gilead Sciences |
| More information | Press release: European Medicines Agency advises on compassionate use of a new combination therapy for chronic hepatitis C |
| Name of medicine | Methysergide-containing products |
|---|---|
| INN | methysergide |
| Marketing-authorisation holder | Amdipharm Mercury and Alliance Pharmaceuticals |
| More information | Methysergide-containing products |
| Name of medicine | Protelos and Osseor |
|---|---|
| INN | strontium ranelate |
| Marketing-authorisation holder | Les Laboratoires Servier |
| More information | Protelos and Osseor |
| Name of medicine | Firazyr |
|---|---|
| INN | icatibant |
| Marketing-authorisation holder | Shire Orphan Therapies GmbH |
| More information | Firazyr: Withdrawn extension application |
| Name of medicine | Heplisav |
|---|---|
| Common name | Hepatitis B (rDNA) vaccine (adjuvanted) |
| Marketing-authorisation holder | Dynavax International B.V. |
| More information | Heplisav: Withdrawn application |