Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 21-24 July 2014
NewsHuman
This page provides an overview of the opinions adopted at the July 2014 meeting of the Committee for Medicinal Products for Human Use (CHMP) and other important outcomes.
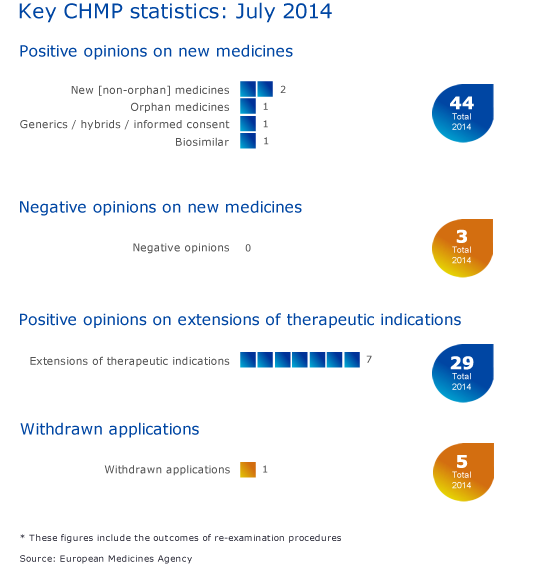
Five new medicines recommended for approval
The CHMP has recommended granting marketing authorisations for two new medicines for the treatment of various rare cancers of the blood, Imbruvica (ibrutinib) and Zydelig (idelalisib). Please see the press release in the grid below for more information.
Granting a marketing authorisation to Xultophy (insulin degludec/liraglutide) for the treatment of diabetes mellitus has also been recommended.
The CHMP recommended granting a marketing authorisation for Accofil (filgrastim), a biosimilar medicine intended for the treatment of neutropenia.
The generic medicine Busulfan Fresenius Kabi (busulfan) was also recommended for authorisation for conditioning treatment prior to conventional haematopoietic progenitor cell transplantation.
Seven recommendations on extensions of therapeutic indications
The Committee recommended extensions of indications for Baraclude, Busilvex, Ecalta, Humira, Ozurdex, RoActemra and Xgeva.
Outcome of review on emergency contraceptives
The CHMP has concluded its review of emergency contraceptives containing levonorgestrel and ulipristal acetate.
Withdrawal of application
The application for a marketing authorisation for Neofordex has been withdrawn. For more information, please see question-and-answer document in the grid below.
Agenda and minutes
The agenda of the July 2014 meeting is published on the EMA website. The minutes of the meeting will be published during the week following the next meeting of the CHMP which will take place from 22-25 September 2014. Minutes of the June 2014 CHMP meeting will be published next week.
CHMP statistics
Key figures from the July 2014 CHMP meeting are represented in the graphic below.
| Name of medicine | Imbruvica |
|---|---|
| International non-proprietary name (INN) | ibrutinib |
| Marketing-authorisation applicant | Janssen-Cilag International NV |
| Therapeutic indication | Treatment of relapsed or refractory mantle cell lymphoma and chronic lymphocytic leukaemia |
| More information | CHMP summary of positive opinion for Imbruvica |
| Press release: European Medicines Agency recommends approval of two new treatment options for rare cancers |
| Name of medicine | Xultophy |
|---|---|
| INN | insulin degludec / liraglutide |
| Marketing-authorisation applicant | Novo Nordisk A/S |
| Therapeutic indication | Treatment of type 2 diabetes mellitus |
| More information | CHMP summary of positive opinion for Xultophy |
| Name of medicine | Zydelig |
|---|---|
| INN | idelalisib |
| Marketing-authorisation applicant | Gilead Sciences International Ltd |
| Therapeutic indication | Treatment of patients with chronic lymphocytic leukaemia and patients with refractory follicular lymphoma |
| More information | CHMP summary of positive opinion for Zydelig |
| Press release: European Medicines Agency recommends approval of two new treatment options for rare cancers |
| Name of medicine | Busulfan Fresenius Kabi |
|---|---|
| INN | busulfan |
| Marketing-authorisation applicant | Fresenius Kabi Oncology Plc |
| Therapeutic indication | Conditioning treatment prior to conventional haematopoietic progenitor cell transplantation |
| More information | CHMP summary of positive opinion for Busulfan Fresenius Kabi |
| Name of medicine | Accofil |
|---|---|
| INN | filgrastim |
| Marketing-authorisation applicant | Accord Healthcare Ltd |
| Therapeutic indication | Treatment of neutropenia |
| More information | CHMP summary of positive opinion for Accofil |
| Name of medicine | Baraclude |
|---|---|
| INN | entecavir |
| Marketing-authorisation applicant | Bristol-Myers Squibb Pharma EEIG |
| More information | CHMP post-authorisation summary of positive opinion for Baraclude |
| Name of medicine | Busilvex |
|---|---|
| INN | busulfan |
| Marketing-authorisation applicant | Pierre Fabre Médicament |
| More information | CHMP post-authorisation summary of positive opinion for Busilvex |
| Name of medicine | Ecalta |
|---|---|
| INN | anidulafungin |
| Marketing-authorisation applicant | Pfizer Limited |
| More information | CHMP post-authorisation summary of positive opinion for Ecalta |
| Name of medicine | Humira |
|---|---|
| INN | adalimumab |
| Marketing-authorisation applicant | AbbVie Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Humira |
| Name of medicine | Ozurdex |
|---|---|
| INN | dexamethasone |
| Marketing-authorisation applicant | Allergan Pharmaceuticals Ireland |
| More information | CHMP post-authorisation summary of positive opinion for Ozurdex |
| Name of medicine | RoActemra |
|---|---|
| INN | tocilizumabum |
| Marketing-authorisation applicant | Roche Registration Ltd |
| More information | CHMP post-authorisation summary of positive opinion for RoActemra |
| Name of medicine | Xgeva |
|---|---|
| INN | denosumab |
| Marketing-authorisation applicant | Amgen Europe B.V. |
| More information | CHMP post-authorisation summary of positive opinion for Xgeva |
| Name of medicine | Emergency contraceptives |
|---|---|
| INN | levonorgestrel / ulipristal acetate |
| More information | Emergency contraceptives |
| Name of medicine | Neofordex |
|---|---|
| INN | dexamethasone acetate |
| Marketing-authorisation applicant | Laboratoires Ctrs - Boulogne Billancourt |
| More information | Questions and answers on the withdrawal of the marketing-authorisation application for Neofordex |