Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 22-25 April 2014
NewsHuman
This page provides an overview of the opinions adopted at the April 2014 meeting of the Committee for Medicinal Products for Human Use (CHMP) and other important outcomes.
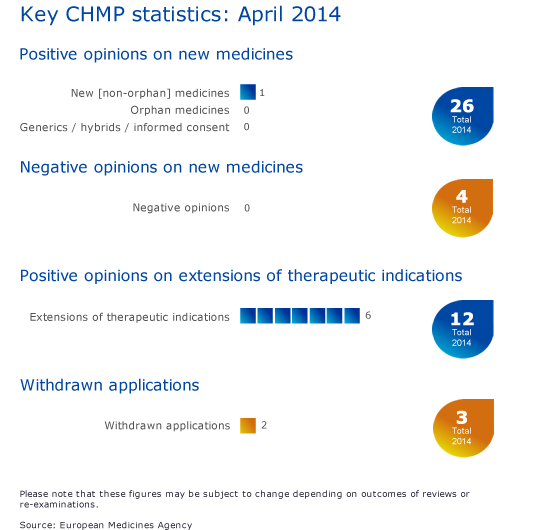
One new medicine recommended for approval
The CHMP has recommended granting a marketing authorisation for Mekinist (trametinib) for the treatment of adult patients with unresectable or metastatic melanoma with a BRAF V600 mutation. Mekinist is the first cancer treatment that selectively targets the MEK protein kinase. Please see the press release in the grid below for more information.
Seven recommendations on extensions of therapeutic indications
The CHMP has recommended adding a new indication to the use of the cancer medicine Nexavar (sorafenib) to treat progressive, locally advanced or metastatic, differentiated thyroid cancer. Please see the press release in the grid below for more information.
The Committee also recommended extensions of indications for Gilenya, Invega, Pradaxa, Prolia, Gardasil and Silgard.
Review of adrenaline auto-injectors started
The CHMP has started a review of adrenaline auto-injectors, which are used as first-aid treatment of anaphylaxis. This review was requested by the United Kingdom (UK) medicines agency following a national review of all adrenaline auto-injector products approved in the UK.
Outcome of safety review
The CHMP recommended that the marketing authorisations for the dental pastes Caustinerf arsenical, Yranicid arsenical and associated names be revoked in the European Union due to concerns over the risk of genotoxic effects and cell death in tissues around the teeth.
Outcome of re-examination of high-dose estradiol
The CHMP has updated recommendations on the use of two high-strength estradiol-containing creams, Linoladiol N and Linoladiol HN.
Withdrawals of applications
The applications for marketing authorisation for Ditelos/Issarlos and for an extension of indication for Votrient have been withdrawn. Please see question-and-answer documents in the grid below.
Update on Herceptin, Alimta and Remicade
The CHMP discussed the recent theft of vials of the anticancer medicine Herceptin 150 mg. The vials, after being stolen in Italy, were tampered with and re-introduced under false credentials into the supply chain in some countries. The Committee agreed upon a direct communication to alert healthcare professionals in concerned Member States.
A document listing the batches of Herceptin (trastuzumab), Alimta (pemetrexed) and Remicade (infliximab) that are the subject of ongoing investigations into the theft of vials of these medicines in Italy, as announced by the EMA on 16 and 17 April, has been published on the EMA website. The list of batches is based on the latest information available. It will continue to be updated as the situation evolves.
Agenda and minutes
The agenda of the April 2014 meeting is published on the EMA website. The minutes of the meeting will be published during the week following the May CHMP meeting. Minutes of the March 2014 CHMP meeting will be published next week.
CHMP statistics
Key figures from the April 2014 CHMP meeting are represented in the graphic below.
| Name of medicine | Mekinist |
|---|---|
| International non-proprietary name (INN) | trametinib |
| Marketing-authorisation applicant | Glaxo Group Ltd |
| Therapeutic indication | Treatment of unresectable or metastatic melanoma with a BRAF V600 mutation |
| More information | CHMP summary of positive opinion for Mekinist |
| Press release: European Medicines Agency recommends approval of Mekinist for the treatment of melanoma |
| Name of medicine | Gardasil |
|---|---|
| INN | human papillomavirus vaccine [types 6, 11, 16, 18] (recombinant, adsorbed) |
| Marketing-authorisation applicant | Sanofi Pasteur MSD, SNC |
| More information | CHMP post-authorisation summary of positive opinion for Gardasil |
| Name of medicine | Gilenya |
|---|---|
| INN | fingolimod |
| Marketing-authorisation applicant | Novartis Europharm Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Gilenya |
| Name of medicine | Invega |
|---|---|
| INN | paliperidone |
| Marketing-authorisation applicant | Janssen-Cilag International N.V. |
| More information | CHMP post-authorisation summary of positive opinion for Invega |
| Name of medicine | Nexavar |
|---|---|
| INN | sorafenib |
| Marketing-authorisation applicant | Bayer Pharma AG |
| More information | CHMP post-authorisation summary of positive opinion for Nexavar |
| Press release: European Medicines Agency recommends extending use of Nexavar to include treatment of differentiated thyroid cancer |
| Name of medicine | Pradaxa |
|---|---|
| INN | dabigatran etexilate |
| Marketing-authorisation applicant | Boehringer Ingelheim International GmbH |
| More information | CHMP post-authorisation summary of positive opinion for Pradaxa |
| Name of medicine | Prolia |
|---|---|
| INN | denosumab |
| Marketing-authorisation applicant | Amgen Europe B.V. |
| More information | CHMP post-authorisation summary of positive opinion for Prolia |
| Name of medicine | Silgard |
|---|---|
| INN | human papillomavirus vaccine [types 6, 11, 16, 18] (recombinant, adsorbed) |
| Marketing-authorisation applicant | Merck Sharp & Dohme Limited |
| More information | CHMP post-authorisation summary of positive opinion for Silgard |
| Name of medicine | Adrenaline auto injectors |
|---|---|
| More information | Adrenaline auto injectors Article-31 referral |
| Name of medicine | Caustinerf arsenical and Yranicid arsenical |
|---|---|
| INN | lidocaine, ephedrine, arsenic trioxide |
| More information | Caustinerf arsenical and Yranicid arsenical |
| Name of medicine | Estradiol (topical use) |
|---|---|
| More information | Estradiol (topical use) |
| Name of medicine | Ditelos / Issarlos |
|---|---|
| INN | strontium ranelate / cholecalciferol |
| Marketing-authorisation applicant | Les Laboratoires Servier |
| More information | Questions and answers on the withdrawal of the marketing-authorisation application for Ditelos (strontium ranelate / cholecalciferol) |
| Name of medicine | Votrient |
|---|---|
| INN | pazopanib |
| Marketing-authorisation applicant | Glaxo Group Ltd |
| More information | Questions and answers on the withdrawal of the application for a change to the marketing authorisation for Votrient (pazopanib) |