Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 23-26 June 2014
NewsHumanCorporate
This page provides an overview of the opinions adopted at the June 2014 meeting of the Committee for Medicinal Products for Human Use (CHMP) and other important outcomes.
Six new medicines recommended for approval
The CHMP has recommended granting a marketing authorisation for Daklinza (daclatasvir) in combination with other medicinal products for the treatment of chronic hepatitis C virus (HCV) infection in adults. Please see the press release in the grid below for more information.
Abasria (insulin glargine) received a positive opinion for a marketing authorisation for the treatment of diabetes mellitus. Abasria is the first biosimilar insulin to be recommended for marketing authorisation in the European Union.
Vizamyl (flutemetamol (18F)) was recommended for the visual detection of amyloid-beta neuritic plaques in the brain.
Triumeq (abacavir sulfate / dolutegravir sodium / lamivudine) received a positive opinion for a marketing authorisation for the treatment of human immunodeficiency virus (HIV) infection in adults and adolescents from 12 years of age weighing at least 40 kg.
The CHMP gave a positive recommendation for Velphoro (mixture of polynuclear iron(iii)-oxyhydroxide, sucrose and starches) for the control of serum phosphorus levels in patients with end-stage renal disease.
The Committee also recommended granting a marketing authorisation for Clopidogrel / Acetylsalicylic acid Teva (clopidogrel / acetylsalicylic acid) for the prevention of atherothrombotic events.
Positive opinion on Vantobra adopted by written procedure
In addition to the positive opinions for the six new medicines adopted at the June 2014 meeting, the EMA would also like to highlight the CHMP's recommendation to grant a marketing authorisation for the hybrid medicine Vantobra (tobramycin), adopted via written procedure on 2 June 2014.
Seven recommendations on extensions of therapeutic indications
The Committee recommended extensions of indications for Avastin, Eliquis, Enbrel, Eylea, Isentress, Kalydeco and Stivarga.
Re-examination of negative opinion on extension of indication for Avastin requested
The marketing-authorisation holder for Avastin has requested a re-examination of the CHMP's negative opinion recommending the refusal of a change to the marketing authorisation for this medicine, adopted at its May 2014 meeting. The change concerned an extension of indication to add treatment of glioblastoma (an aggressive type of brain cancer). Upon receipt of the grounds of the request for re-examination, the CHMP will re-examine this opinion and issue a final opinion.
Withdrawals of applications
The applications for marketing authorisation for Faldaprevir Boehringer Ingelheim (faldaprevir), and for an extension of indication for Tasigna (nilotinib) have been withdrawn. For more information, please see question-and-answer documents in the grid below.
Agenda and minutes
The agenda of the June 2014 meeting is published on the EMA website. The minutes of the meeting will be published during the week following the July CHMP meeting. Minutes of the May 2014 CHMP meeting will be published next week.
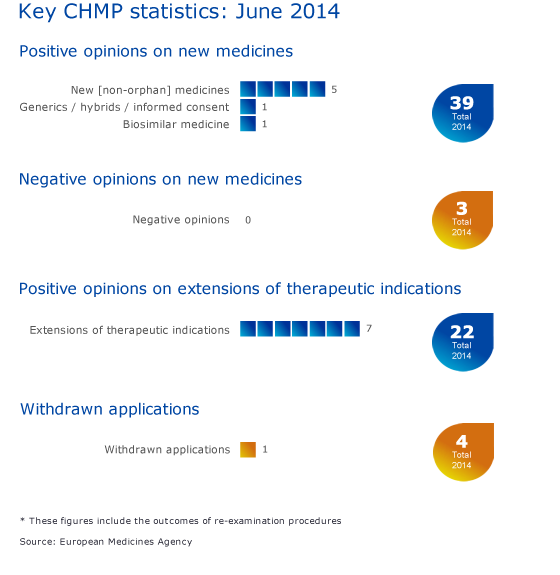
CHMP statistics
Key figures from the June 2014 CHMP meeting are represented in the graphic below. The total number of positive new opinions includes the hybrid medicine Vantobra, which was adopted by the Committee by written procedure in June 2014.
| Name of medicine | Daklinza |
|---|---|
| International non-proprietary name (INN) | daclatasvir |
| Marketing-authorisation applicant | Bristol-Myers Squibb Pharma EEIG |
| Therapeutic indication | Treatment of chronic hepatitis C virus (HCV) |
| More information | |
| Press release: European Medicines Agency recommends approval of Daklinza in chronic hepatitis C |
| Name of medicine | Triumeq |
|---|---|
| INN | abacavir sulfate / dolutegravir sodium / lamivudine |
| Marketing-authorisation applicant | ViiV Healthcare Uk Limited |
| Therapeutic indication | Treatment of Human Immunodeficiency Virus (HIV) infection in adults and adolescents from 12 years of age weighing at least 40 kg |
| More information | CHMP summary of positive opinion for Triumeq |
| Name of medicine | Velphoro |
|---|---|
| INN | mixture of polynuclear iron(iii)-oxyhydroxide, sucrose and starches |
| Marketing-authorisation applicant | Vifor Fresenius Medical Care Renal Pharma France |
| Therapeutic indication | Indicated for the control of serum phosphorus levels in patients with end-stage renal disease |
| More information | CHMP summary of positive opinion for Velphoro |
| Name of medicine | Vizamyl |
|---|---|
| INN | flutemetamol (18F) |
| Marketing-authorisation applicant | GE Healthcare Ltd |
| Therapeutic indication | Indicated for the visual detection of amyloid-beta neuritic plaques in the brain |
| More information | CHMP summary of positive opinion for Vizamyl |
| Name of medicine | Clopidogrel / Acetylsalicylic acid Teva |
|---|---|
| INN | clopidogrel / acetylsalicylic acid |
| Marketing-authorisation applicant | Teva Pharma B.V. |
| Therapeutic indication | Prevention of atherothrombotic events |
| More information | CHMP summary of positive opinion for Clopidogrel / Acetylsalicylic acid Teva |
| Name of medicine | Abasria |
|---|---|
| INN | insulin glargine |
| Marketing-authorisation applicant | Eli Lilly Regional Operations GmbH |
| Therapeutic indication | Treatment of diabetes mellitus |
| More information | CHMP summary of positive opinion for Abasria |
| Name of medicine | Avastin |
|---|---|
| INN | bevacizumab |
| Marketing-authorisation applicant | Roche Registration Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Avastin |
| Name of medicine | Eliquis |
|---|---|
| INN | apixaban |
| Marketing-authorisation applicant | Bristol-Myers Squibb / Pfizer EEIG |
| More information | CHMP post-authorisation summary of positive opinion for Eliquis |
| Name of medicine | Enbrel |
|---|---|
| INN | etanercept |
| Marketing-authorisation applicant | Pfizer Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Enbrel |
| Name of medicine | Eylea |
|---|---|
| INN | aflibercept |
| Marketing-authorisation applicant | Bayer Pharma AG |
| More information | CHMP post-authorisation summary of positive opinion for Eylea |
| Name of medicine | Isentress |
|---|---|
| INN | raltegravir |
| Marketing-authorisation applicant | Merck Sharp & Dohme Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Isentress |
| Name of medicine | Kalydeco |
|---|---|
| INN | ivacaftor |
| Marketing-authorisation applicant | Vertex Pharmaceuticals (UK) Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Kalydeco |
| Name of medicine | Stivarga |
|---|---|
| INN | regorafenib |
| Marketing-authorisation applicant | Bayer Pharma AG |
| More information | CHMP post-authorisation summary of positive opinion for Stivarga |
| Name of medicine | Seasonique and associated names |
|---|---|
| INN | levonogestrel 150 ?g and ethinylestradiol 30 ?g / 10 ?g |
| More information | Questions and answers on Seasonique and associated names |
| Name of medicine | Sandostatin and associated names |
|---|---|
| INN | octreotide acetate |
| More information | Questions and answers on Sandostatin and associated names |
| Name of medicine | Sandostatin LAR and associated names |
|---|---|
| INN | octreotide acetate |
| More information | Questions and answers on Sandostatin LAR and associated names |
| Name of medicine | Faldaprevir Boehringer Ingelheim |
|---|---|
| INN | faldaprevir |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Faldaprevir Boehringer Ingelheim (faldaprevir) |
| Name of medicine | Tasigna |
|---|---|
| INN | nilotinib |
| Marketing-authorisation applicant | Novartis Europharm Ltd |
| More information | Questions and answers on the withdrawal of the application for a change to the marketing authorisation for Tasigna (nilotinib) |