Variations not requiring assessment (veterinary medicines)
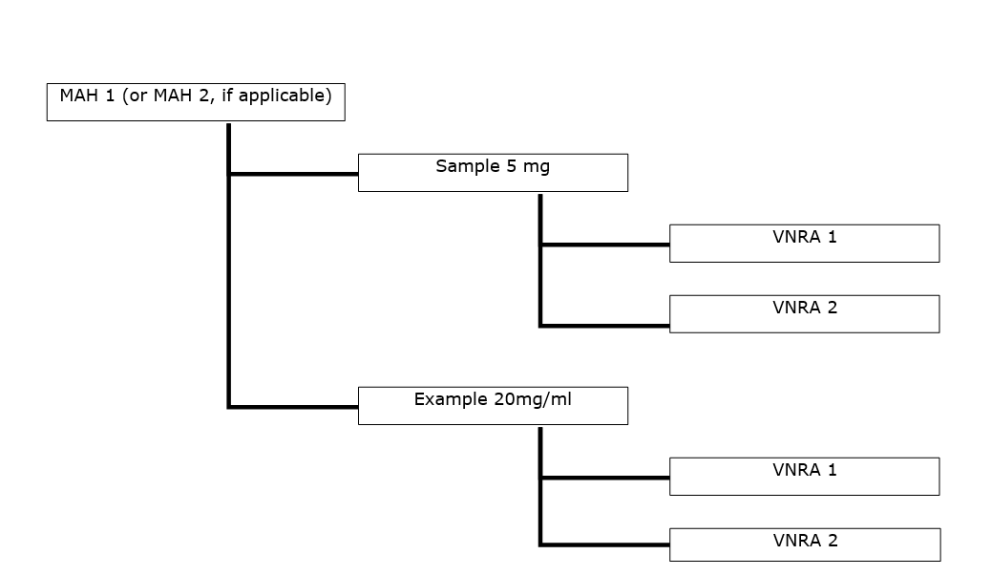
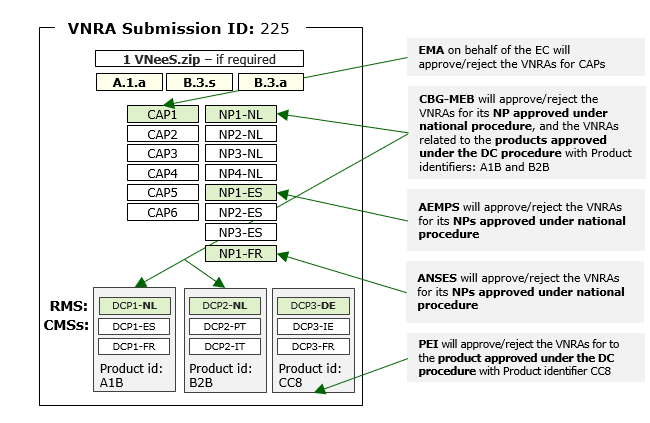
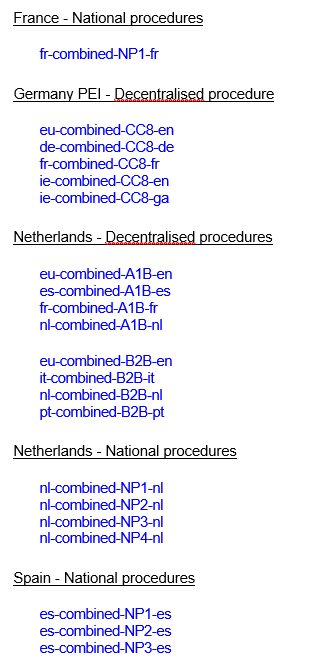
Guidance is available from the European Medicines Agency (EMA) on variations for centrally authorised veterinary medicines not requiring assessment under the Veterinary Medicinal Products Regulation (Regulation (EU) 2019/6). This includes information on the documents that marketing authorisation holders need to submit as part of the variation procedure.
VeterinaryRegulatory and procedural guidance