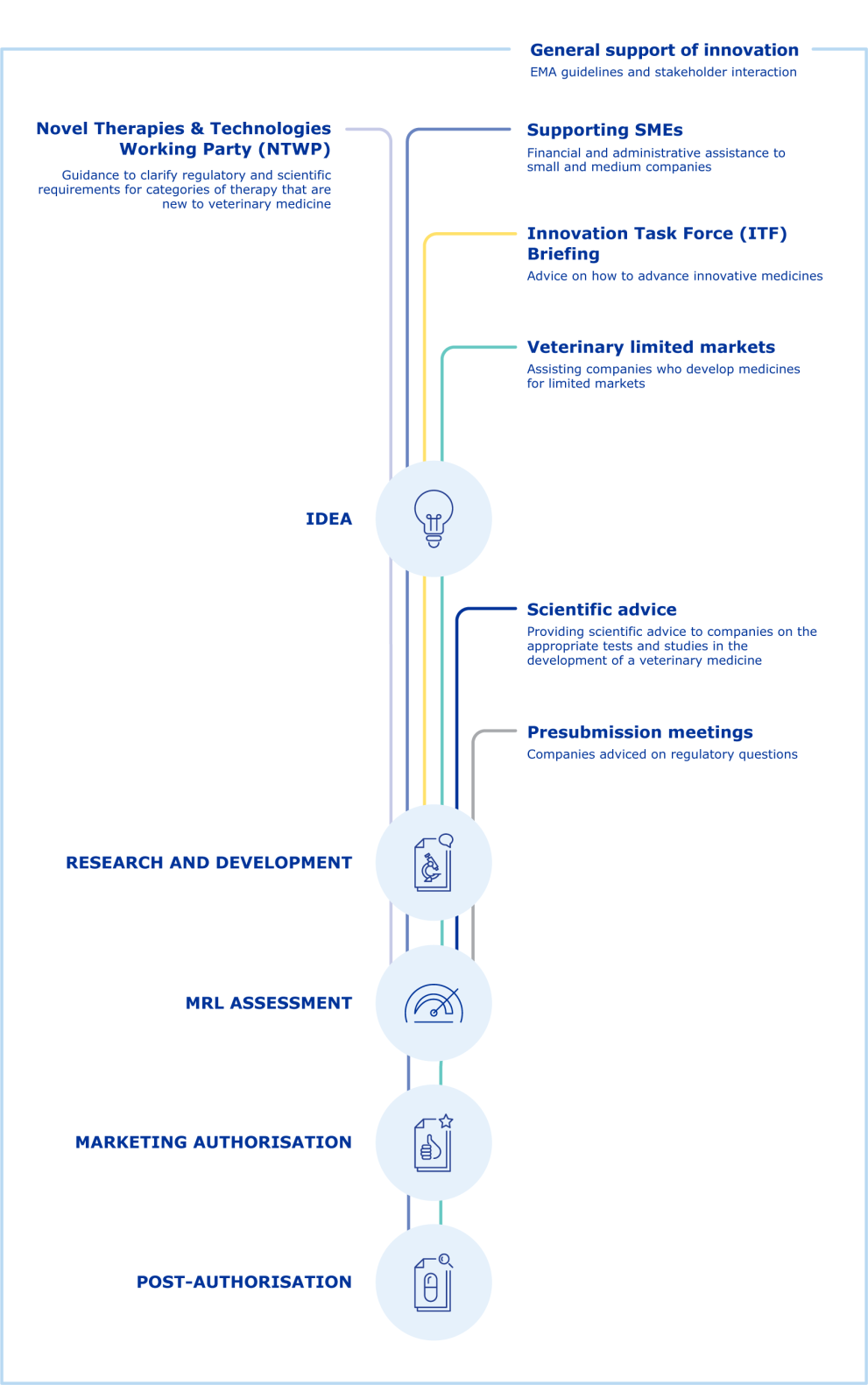
Innovation in veterinary medicines
One of the European Medicines Agency's (EMA) strategic goals is to promote development of innovative veterinary medicines and new technologies. Technologies that are new to veterinary medicine present particular challenges due to a lack of regulatory guidance and, in some cases, the fact that the existing regulatory framework does not specifically cater for them.
VeterinaryInnovation