Authorisation of medicines
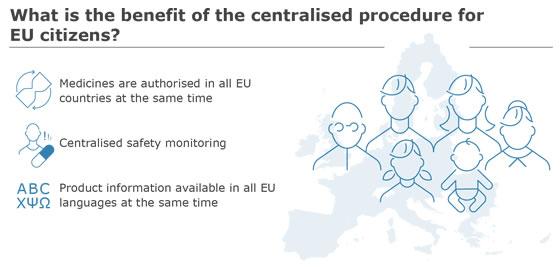
All medicines must be authorised before they can be marketed and made available to patients. In the European Union (EU), there are two main routes for authorising medicines: a centralised route and a national route.
Corporate