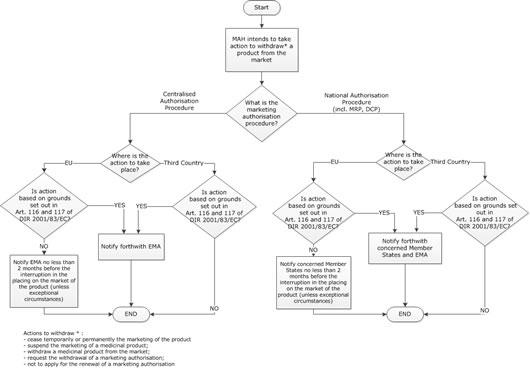
Notifying a change of marketing status
This page lists questions relating to the notification of marketing and cessation, suspension, withdrawal of a medicinal product from the market and withdrawal of a marketing authorisation. It also covers 'sunset-clause' monitoring.
HumanRegulatory and procedural guidance