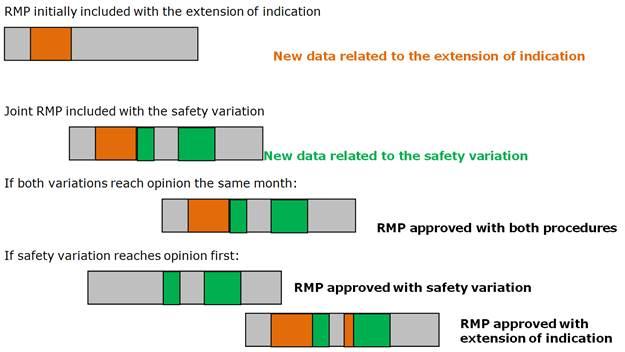
Risk management plans (RMP) in post-authorisation phase: questions and answers
This page is intended to provide advice to marketing authorisation holders of centrally authorised medicinal products about procedural and regulatory aspects to the risk management plan (RMP) lifecycle during the post authorisation phase.
HumanRegulatory and procedural guidancePharmacovigilance