Post-authorisation measures: questions and answers
This page lists questions that marketing-authorisation holders (MAHs) may have on post-authorisation measures.
Updated on 26 November 2025:
- to update PDF versions of questions and answers and questions marked with "Rev. Nov 2025"
This page provides an overview of the European Medicines Agency's (EMA's) position on issues that are typically addressed in discussions or meetings with MAHs in the post-authorisation phase. Revised topics are marked 'New' or 'Rev.' upon publication.
These questions and answers have been produced for guidance only and should be read in conjunction with the rules governing medicinal products in the European Union, volume 2, notice to applicants.
MAHs must in all cases comply with the requirements of Community legislation. Provisions that extend to Iceland, Liechtenstein and Norway by virtue of the European Economic Area agreement are outlined in the relevant sections of the text.
From January 2025, marketing authorisation holders should use the IRIS platform when managing post-authorisation measures after the original submission.
Further guidance on the use of the IRIS platform and how to prepare submissions is available on the dedicated IRIS website:
IRIS does not replace the current submission gateway; they coexist serving different functions.
At the time of finalising a procedure or in follow-up of a signal evaluation, the Agency's Committee(s) may agree that the applicant/MAH should provide additional data post-authorisation, as it is necessary from a public health perspective to complement the available data with additional data about the safety and, in certain cases, the efficacy or quality of authorised medicinal products. Such post-authorisation measures (PAMs) may be aimed at collecting or providing data to enable the assessment of the safety or efficacy of medicinal products in the post-approval setting.
The existence of such a system of PAMs does not aim at promoting premature approvals of marketing authorisations or post-authorisation procedures. The background and rationale for requesting PAMs will be described in the relevant assessment, which will present the context and nature of the PAM. Based on the assessment of the committee(s), PAMs are classified into their appropriate legal framework under which they will be enforced.
The following diagram explains how PAMs are categorised; in addition, each PAM category is explained in the following sections:
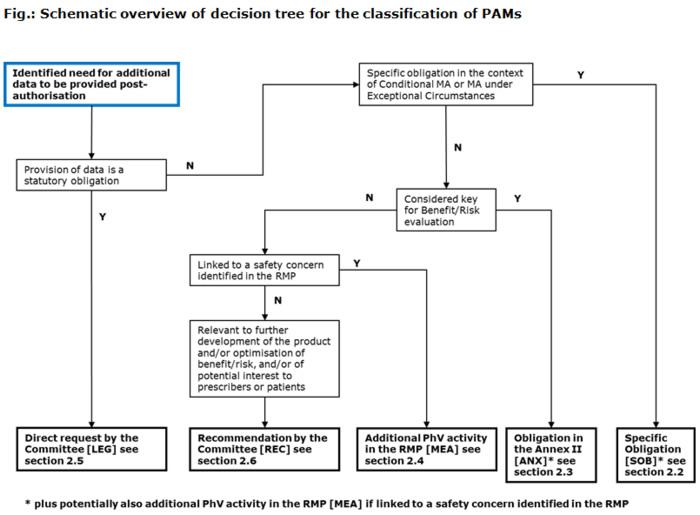
Consequently, PAMs fall within one of the following categories [EMA codes1]:
Only certain medicinal products can be subject to specific obligations (see also 'What is a Specific Obligation?'). PAMs other than specific obligations can be required for any type of authorisation and will be included in the opinion of an initial marketing authorisation or further to the committees' assessment during post-authorisation.
The wording of the PAM will describe the issue under investigation that has led to the request together with a clear outline of the studies or activities expected to address it and the deadline for its submission. Compliance with these measures is defined by both the submission of the requested data and adherence to the agreed timeframe.
References
1These codes relate to the Agency's product and procedure tracking database called SIAMED and will be used, together with a numbering system, to identify each PAM of a medicinal product both in the database and in any correspondence of the Agency with the MAH.
Specific obligations can only be imposed on marketing authorisations granted under exceptional circumstances or on conditional marketing authorisations (see also questions on 'Is my medicinal product eligible for approval under exceptional circumstances?' and 'Could my application qualify for a conditional marketing authorisation?' of the Agency's presubmission guidance). These are conditions to the marketing authorisation included in annex II.E of the Commission decision and form the basis of the annual re-assessment or the annual renewal. These may also be additional Pharmacovigilance activity and will be included as well in the RMP (category 2 studies).
Continuation of a marketing authorisation under exceptional circumstances or the renewal of a conditional marketing authorisation will be determined by the MAH's compliance with the specific obligations, which are checked annually as part of either the annual reassessment or the annual renewal procedures.
As specific obligations are binding conditions to the marketing authorisation, any modification proposal by the MAH with regards to their description or due date (as described in Annex II of the product information) has to be submitted within an appropriate procedure, i.e. either within the annual re-assessment, the annual renewal or a variation application.
Interim results not impacting on the product information or on the description of the specific obligation can be submitted as a PAM as described below, if they are not part of the annual reassessment or annual renewal. (see: How and to whom shall I submit my PAM data?).
In case of interim results impacting on the product information, a variation should be submitted without waiting for the annual re-assessment or annual renewal.
Final results leading to the fulfilment of the specific obligation should be submitted within an appropriate procedure, i.e. either within the annual re-assessment, the annual renewal or a variation application.
Where a specific obligation falls within the definition of a non-interventional post-authorisation safety study (PASS) imposed after 2 July 2012, the MAH will have to follow the procedure for review of imposed PASS protocols and results as described in the Agency's post-authorisation procedural advice on PASS and in the corresponding Guideline on good pharmacovigilance practices (GVP) - Module VIII – Post-authorisation safety studies (Rev. 3).
References
The European Commission can impose on the marketing authorisation holder (MAH) the obligation to conduct post-authorisation measures. These obligations can be imposed at the time of the granting of the marketing authorisation or later, as conditions to the marketing authorisation. These are conditions to the marketing authorisation included in Annex II.D of the marketing authorisation. These may also be additional Pharmacovigilance activity and will be included in the RMP (category 1 studies).
Annex-II conditions are post-authorisation measures which, whilst not precluding the approval of a marketing authorisation or other post-authorisation procedures, are considered to be key to the benefit / risk balance of the product. These can consist of post-authorisation safety or efficacy study.
As annex-II obligations are binding conditions to the marketing authorisation, any modification proposal by the MAH with regards to their description or due date has to be submitted as a variation application.
Interim results not impacting on the product information or on the condition as stated in the Annex II can be submitted as a PAM as described in question How and to whom shall I submit my PAM data?.
Final results leading to the fulfilment of the Annex II condition should be submitted as a variation application.
Where an annex-II condition falls within the definition of a non-interventional PASS imposed after 2 July 2012, the MAH will have to follow the procedure for review of imposed PASS protocol and results as described in the Agency's post-authorisation procedural advice on PASS and in the corresponding Guideline on good pharmacovigilance practices (GVP) - Module VIII – Post-authorisation safety studies (Rev. 3).
References
Additional pharmacovigilance activities in the RMP (category 3 studies) may be non-clinical studies, clinical trials or non-interventional studies which are required to investigate a safety concern of a medicinal product. These studies are listed in the pharmacovigilance plan of the risk-management plan (RMP) and are either aimed at identifying and characterising risks, or at assessing the effectiveness of risk-minimisation activities.
All relevant milestones, together with their due dates should be included in the summary table of additional PhV activities in the RMP. The MAH has the obligation to provide the requested data within the stated timeframes.
Once additional pharmacovigilance activities have been agreed within the RMP, changes to these measures (e.g. proposals for adjusting due dates of agreed milestones, proposals to change the scope of agreed study or its duration, etc.) should be submitted via the appropriate variation procedure to amend the RMP.
Information not impacting on the product information or description/due date of the measure itself, (e.g. interim results) , can be submitted as a self-standing PAM as described in question How and to whom shall I submit my PAM data?.
Submissions of final study reports leading to the fulfilment of a MEA should be addressed via the appropriate variation procedure. (see also: Under which procedure should I submit my PAM?).
References
Some post-authorisation measures (PAMs) are already defined as statutory obligations in the pharmaceutical legislation. As such, they have to be fulfilled by the MAH upon request of the Agency and its committees. Examples for such directly binding legal measures evaluated as PAMs are:
Where requested, these are directly addressed to the MAH by the Agency, either within the assessment report of the committee(s) or within a letter informing about the Committee(s)’(s) conclusions and have to be responded to within the stated time frame.
Requests for updates of the product information should be addressed via a variation; a scientific justification for not submitting a requested variation should be submitted as a PAM.
When responding to these requests, the MAH should select “pam-leg” as submission type when filling in the eSubmission delivery file except for:
In accordance with the Paediatric legislation, MAHs should submit paediatric studies within six months of their completion and irrespective of whether it is part of a PIP (completed or not yet completed) or not, or whether it is intended for submission later on as part of a variation, extension or new stand-alone marketing-authorisation application.
References
During the assessment of an application, the committee(s) may issue recommendations for further development of the medicinal product, e.g. either in terms of optimising some quality aspects or considerations for extending the patient population. Although these recommendations for further development are not binding to the marketing authorisation, they should be seen as important considerations in view of the potential future use of a medicinal product by the MAH.
This information can be submitted as a PAM however if data obtained in the framework of a recommendation has an impact on the authorised medicinal product and its product information, the MAH has the obligation to submit a variation application as appropriate (see: How and to whom shall I submit my PAM data?).
As such, the committee(s) will keep an overview of all recommendations made to a marketing authorisation and monitor whether, how and when the MAH has addressed them. Therefore, MAHs are encouraged to use the Letter of recommendations - Template to acknowledge these recommendations.
MAHs should specify the following in their letter of recommendations:
No deadline needs to be mentioned.
When data in relation to a recommendation is provided to the Agency, an updated Letter of Recommendation should be provided, in which the MAH should indicate the date of submission and its format (e.g. as self-standing data, within a variation, within a renewal etc.).
References
New data or information regarding the medicinal product becoming available can result in the committee(s) considering that a PAM should be reclassified. Such reclassification will be performed within the procedure discussing the impact of the new information that has become available and will be justified in the assessment report where the measure is, as a consequence, up- or downgraded.
The MAH shall submit the PAM data according to the timeframe specified by the Agency's committee(s) as specified either in the annex II, the RMP or the respective committee assessment. When requested, the MAHs should propose due dates for the submission of the post-authorisation data that are realistic and proportionate to the uncertainty to be addressed which are then subject to agreement with the Agency's committees .
Data submitted as PAM should be submitted as per the deadline specified by the Committee(s), and will start in accordance with the published submission dates for PAMs (see also Human Medicines - Procedural timetables / Submission dates). Assessment of PAM data submitted after the recommended submission date will start in accordance with the start date of the following month.
If the MAH is unable to provide the required data by the specified deadline, he must inform the Agency and the rapporteur in writing as early as possible in advance of the due time of submission. The reason for the delay must be justified and a new submission date proposed and is subject to agreement by the Committee(s). These submissions should be done as follows:
References
The procedure under which the PAM should be submitted will depend on the content and type of information submitted as part of the PAM, as summarised in the table below:
| PAM | Submission | Procedure/Type of application |
| Specific obligation (category 2) [SOB] |
Non-interventional PASS
|
See, Post-authorisation safety studies: questions and answers
|
| Annex II E Interventional Efficacy Studies | ||
|
Stand-alone PAM [SOB] Where a protocol is not requested to be submitted by the Agency's Committee, the MAH should consider to seek scientific advice |
|
|
Conditional renewal, annual re-assessment (Note: if submission of interim results is requested outside of the timelines of the renewal or annual re-assessment, these can be submitted as stand-alone PAM, if no changes to the PI are proposed), alternatively a type II would be required. |
|
|
Conditional renewal, annual re-assessment or type II variation, depending on the timelines. | |
| Annex II condition (category 1) [ANX] |
Non-interventional PASS | See, Post-authorisation safety studies: questions and answers |
|
Article 107n-o | |
|
ANX | |
|
Article 107p-q | |
| Type II variation | Other studies: Final results | Type II variation |
| Additional Pharmacovigilance activity in the RMP (category 3) [MEA] |
Protocol (as requested by Committee and reflected as a milestone in the RMP) |
Stand-alone PAM [MEA] Where a protocol is not requested to be submitted by the Agency's Committee, the MAH should consider to seek scientific advice |
|
Interim results
|
Stand-alone PAM [MEA] | |
|
Type II variation | |
| Final results | Type II variation | |
| Additional Pharmacovigilance activity in the RMP (category 3) [MEA] |
Provision of data requested by the Committee (e.g. cumulative review, CAPA, interim study results) ([SDA] when related to a signal assessment) |
|
|
Stand-alone PAM [LEG]/[SDA] | |
|
Type II variation | |
| Final study report | Type II variation | |
|
Justification for not submitting a variation ([SDA] when related to a signal assessment, otherwise [LEG]) |
Stand-alone PAM [LEG]/[SDA] | |
| Submission of final results of study involving paediatric patients in accordance with Article 46 of the Paediatric Regulation [P46] | ||
|
Stand-alone PAM [P46] | |
|
Type II variation | |
| Recommendation [REC] |
Interim results | |
|
Stand-alone PAM [REC]
|
|
|
Type II variation | |
| Final results | Type II variation | |
| ERA study results with no impact to PI | Type IB CI.z/C.z variation | |
| Recommendation [REC] - Quality |
No changes introduced to Module 3 (confirmatory data e.g. confirmatory stability data (without claiming a change in shelf-life/ in-use shelf-life or storage conditions) | Stand-alone PAM [REC] |
| Changes introduced to Module 3 | Respective variation as per the Variation Classification Guideline |
Where the deliverable of a measure is submitted as part of another procedure, the structure of the submission package should follow the requirements of this procedure and the MAH should indicate in the cover letter of the application which PAM is being addressed, including the EMA reference number and the full description of the relevant PAM. The PAM submission form does not need to be included in the variation submission package. The MAH does not need to submit a separate ‘stand-alone’ submission of the PAM data.
References
The Agency will check PAM submissions with respect to the Guidelines on Variations to ensure that it does not fall within one of the classifications. In this regard, the Agency will reject any PAM submission that should be filed as a variation application. In such cases, the eCTD submission of the variation application should provide a reference to the PAM eCTD submission for this sequence to be closed. Where the MAH is requested to resubmit as a variation application, the start of the variation procedure will be upon receipt of the complete application according to the next upcoming starting date as per published timetable for Type II.
’Stand-alone’ PAM submission must include:
References
Information is available on ‘Submitting a post-authorisation application’.
This section only applies to submissions of PAM data as a 'stand-alone' submission.
Most PAMs will be evaluated by CHMP (and CAT if an advanced therapy medicinal product).
However, PRAC will lead the review of protocols or interim results of non-interventional safety studies and in any follow-up PAM to a procedure primarily assessed by PRAC (e.g: cumulative safety review requested further to the assessment of PSUR [LEG] or a signal [SDA]).
PAMs will be handled using one of the three timetables:
The submission deadlines and full procedural detailed timetables are published as a standard calendar on the EMA website (see: Human Medicines – Procedural Timetables / Submission dates).
The Agency will send to the MAH the final assessment report after CHMP adoption. The following outcome may be envisaged depending on the committee’s conclusion:
References
There is no fee payable for a PAM stand-alone submission.
The Agency will keep a record of the post-authorisation measure and its due date in its database. In addition, the compliance with specific obligations is assessed annually, as part of the annual renewal (for conditional marketing authorisations) or annual reassessment (for marketing authorisations under exceptional circumstances).
In case of overdue measures or a MAH being found non-compliant in satisfying a post-authorisation measure, the responsible committee will consider the need for appropriate actions to be taken including involvement of the relevant committee(s).
In such situations, the rapporteur (or a lead rapporteur nominated by the committee in case of more than one affected product) may draft an assessment report on the impact of the lack of data on the benefit/risk balance of the affected product or other analysis to support a discussion on the next steps by the Agency's committee(s). Based on the outcome of such assessment and/or discussion, one or more of the following actions may be taken:
Furthermore, according to Article 20a of Regulation (EC) No 726/2004, a conditional marketing authorisation shall be varied, suspended or revoked if it is concluded that that the MAH has failed to comply with the obligations laid down in the conditional marketing authorisation.
Such regulatory action in regards to non-compliance of a MAH may be made public by the Agency on the Agency website e.g. in the EPAR(s) of the affected product(s).
Irrespective of the above regulatory actions, the Agency may take at any point in time a decision to take another enforcement action beyond those described here.
References
Outcome of PAMs are not published in the EPAR 'Procedural steps taken and scientific information after the authorisation'. However, assessment reports for data submitted in accordance with Article 46 of the Paediatric Regulation and PRAC recommendations on signals are published on the Agency's website.
Reference
If you cannot find the answer to your procedural question in the post-authorisation measures: question and answers when preparing your application, please contact your Product Lead.
Information is available on ‘Submitting a post-authorisation application’.