Grouping of variations: questions and answers
This page lists questions that marketing-authorisation holders (MAHs) may have on grouping of variations.
HumanRegulatory and procedural guidance
Updated on 26 November 2025:
- to update PDF versions of questions and answers and questions marked with "Rev. Nov 2025"
This page provides an overview of the European Medicines Agency's position on issues that are typically addressed in discussions or meetings with MAHs in the post-authorisation phase. Revised topics are marked 'New' or 'Rev.' upon publication.
These questions and answers have been produced for guidance only and should be read in conjunction with the rules governing medicinal products in the European Union, volume 2, notice to applicants.
MAHs must in all cases comply with the requirements of Community legislation. Provisions that extend to Iceland, Liechtenstein and Norway by virtue of the European Economic Area agreement are outlined in the relevant sections of the text.
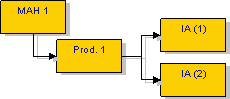
Marketing authorisation holder can group several Type IA/ IAIN variations to the terms of the same marketing authorisation under a single notification to the same relevant authority:
Several
This means for instance that a
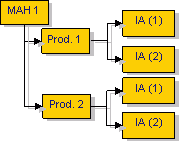
Article 7a of the Variations Regulation, as amended, sets out the possibility for a marketing authorisation holder to super-group the same or several Type IA/ IAIN variations to the terms of more than one marketing authorisation under a single notification to the same relevant authority:
one

several
Applicants belonging to the same mother company or group of companies and applicants having concluded agreements or exercising concerted practices concerning the placing on the market of the medicinal product(s) concerned, have to be taken as “the same marketing authorisation holder”.1
Please note that currently it is not operationally possible to have super-grouping of Type IA variations including simultaneously marketing authorisations approved via the centralised procedure and non-centralised procedure. Additional cases taking into account the experience acquired may be identified in the future and appropriate operational guidance will be provided in Agency and CMDh websites accordingly.
Articles 7.2(b) and 7.2(c) of the Variations Regulation set-out the possibility for a marketing authorisation holder to group several types of variations affecting one medicinal product, under a single notification/application.
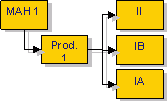
Article 7.2(b) applies for groupings that are listed in Annex III of the Regulation whilst article 7.2(c) applies for groupings of variations which are not listed in Annex III, but which have been agreed with the Agency.
In the case of groupings under Article 7.2(c) it is recommended that the grouping is agreed between the holder and the Agency at least 2 months before submission.
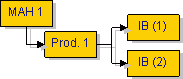
Where the same Type IB or Type II variation, or group of variation(s) affect several medicinal products from the same MAH, the MAH shall submit these variations as one application for ‘worksharing’. Please also refer to “What is worksharing and what types of variations can be subject to worksharing?”
References
1 See Commission Communication 98/C 229/03 OJ C 229, 22.7.1998, p. 4.
Related Type
It must be noted however, that when submitting Type IA/ IAIN variations as part of a group, the legal deadlines for submission of each variation should be respected i.e. a Type IAIN should always be submitted immediately, whether or not it is grouped with other variations, and any Type IA variation should always be submitted within 12 months following its implementation.
For further information see also When shall I submit my Type IA/IAIN variation(s).
When super-grouping one or more
Grouping of non-Type IA variations is only acceptable when they fall within one of the cases listed in Annex III of the Regulation, or, if they do not fall within one of those cases, when the grouping of the variations has been agreed between the Agency and the MAH before submission.
MAHs are advised to inform the Agency at least 2 months in advance of the submission of a group of variations which are not listed in Annex III of the Regulation, together with a justification as to why the holder believes that the proposed group should be acceptable.
When reviewing MAH proposals for grouping of variations, the Agency will consider the following general principles:
Table 1 presents some examples of acceptable groups of variations listed in Annex III of the Regulation, with further clarification on how such groups will be considered in practice.
Table 2 presents some examples of other groups of variations, which the Agency would or not in principle consider acceptable. Some concrete examples are also provided to illustrate proposed groupings that could be considered acceptable or not.
These tables will be reviewed and updated regularly, in view of accumulated experience.
Table 1. Grouping examples according to Article 7.2(b) of the Variation Regulation (Cases for grouping variations listed in Annex III)
| 1 | One of the variations in the group is an extension of the marketing authorisation. | Other clinical or non-clinical changes linked to the extension (e.g. a new indication) can be grouped with the Extension application. Quality changes affecting the drug substance and/or drug product can also be included in the group. |
| Example: Extension of the marketing authorisation for a new strength/pharmaceutical form + type II variation for new therapeutic indication concerning the already authorised strength(s)/ pharmaceutical form(s).ccc | ||
| 2 | One of the variations in the group is a major variation of type II; all other variations in the group are variations which are consequential to this major variation of type II. | “A consequential variation is regarded as a change, which is an unavoidable and direct result of another change (i.e. the 'main change') and not simply a change which occurs at the same time.” |
| Example: type II for new indication + type IB or IA for addition of a new pack size required for the use in this new indication. Grouping of non-consequential quality changes may also be acceptable, under Article 7.2(c) other groups to be agreed with the Agency. | ||
Table 2. Grouping examples according to Article 7.2(c) of the Variation Regulation (Cases for grouping variations agreed by the Agency)
| 1 | Grouping of variations relating to active substance or finished product (but not to both) |
Grouping acceptable
|
| Example: type IB - extension of re-test period of the active substance + type IB - changes in the storage conditions of the active substance. | ||
| 2 | Grouping of variations relating to active substance and linked variations relating to finished product |
Grouping acceptable
|
| Example: type IB - changes to a test procedure of the active substance + type IA - deletion of a non-significant in-process control of the finished product. | ||
| 3 | Grouping of quality and administrative variations | Grouping acceptable (administrative change can be combined with quality change when PI Annexes are affected). |
| Example: type IB - extension of the shelf life of the finished product + type IA(IN) - change in the name of a manufacturer responsible for batch release + type IA - change in ATC Code. | ||
| 4 | Grouping of several non-clinical studies | Grouping acceptable. |
| Example: Provision of final study reports for 7 non-clinical in vivo studies, one of which results in consequential changes to the SmPC. The study report affecting the PI should be submitted as part of one Type II variation under category C.I.4/C.4* and the remaining 6 reports as part of 6 Type II variations under category C.I.13/C.12* (one variation per study report). As all 7 studies are non-clinical the scopes are related, and it is considered meaningful for these variations to be reviewed simultaneously. Thus, the MAH should submit one grouped application including one Type II variation under category C.I.4/C.4* and six Type II variations under category C.I.13/C.12*. | ||
| 5 |
Grouping of several drug-drug interaction studies e.g. type II - interaction study with Rifampicin + type II- interaction study with oral contraceptive |
Grouping acceptable; 1 type II variation scope per interaction study, but type II variations can be grouped in 1 application. |
| 6 | Grouping of several safety changes with similar implementation timelines | Grouping acceptable, provided that the variations are to be led by the same committee |
|
Example 1: Update of section 4.4 of the SmPC with regard to venous thromboembolic events and haemorrhage events, and update of section 4.8 of the SmPC to include unrelated new ADRs, all following an update of the MAH’s product core safety data sheet based on three different sets of data. The addition of information on venous thromboembolic events to SmPC section 4.4 is based on the analysis of one data set and requires one Type II variation under category C.I.4/C.4*. The addition of information on haemorrhage events to SmPC section 4.4 is based on the analysis of another data set and therefore requires one additional Type II variation under category C.I.4/C.4*. The addition of the new ADRs is in this case not consequential to the changes to SmPC section 4.4 above and is supported by another data set. Thus, the addition of the new ADRs to SmPC section 4.8 constitutes one additional scope and will therefore require an additional variation under category C.I.4/C.4*. The applicant should in this case submit one grouped application including 3 Type II variations under category C.I.4/C.4*. The three variations are all related to clinical safety, they will be assessed by the CHMP and a common assessment is expected and is consequentially meaningful. |
||
|
Example 2: Update of section 4.8 of SmPC to add three new ADRs - dyspnoea and chromaturia following a review of the MAH’s safety database upon request by PRAC following a PSUSA procedure and Kounis syndrome following the MAH’s own signal detection. As the three ADRs are supported by two separate data sets the MAH should submit two variations; one Type II variation under category C.I.3.b/C.3.c* to add dyspnoea and chromaturia and one Type II variation under category C.I.4/C.4* to add Kounis syndrome. Both variations are related to clinical safety, but the assessment of the first variation is to be led by the PRAC while that of the second one will be performed by the CHMP; hence, the grouping is not acceptable in this case. |
||
| 7 | Grouping of several variations affecting the product information with different recommended or expected approval timelines | Grouping not acceptable |
|
Example 1: Type IA(IN) to implement the outcome of signal assessment and type II safety variation. The implementation of the signal recommendation (which includes all language translations) is meant to allow the immediate implementation of the updated Product Information wording. Grouping with a type II variation would delay the implementation, therefore this is not acceptable. |
||
|
Example 2: Type IB variation to implement agreed wording in the Product Information and type II (non) clinical variation. In principle, the grouping is not acceptable as it would delay the implementation of the agreed wording due to longer timelines and possible need for linguistic review for the type II variation. |
||
|
Example 3: Type II variation to propose an extension of the authorised indication. In addition, the applicant proposes an update of the SmPC regarding hepatotoxicity based on a review of the MAH’s safety database undertaken upon request by the CHMP following a previous PAM assessment, and an update of section 4.4 of the SmPC regarding pulmonary toxicity following a literature review. Given the long assessment timelines for an extension of indication application and the fact that a grouped approach would delay the implementation of new safety information, the proposed grouping would not be acceptable. Hence, the extension of indication application should be submitted as a separate stand-alone Type II variation under category C.I.6.a/ C.6.a*. As the two safety topics are supported by different sets of data they should be submitted as part of two separate Type II variations under category C.I.4/C.4*. However, as both scopes concern clinical safety they can be submitted as one grouped application. Thus, the applicant should submit one stand-alone Type II variation under category C.I.6.a/ C.6.a* and one grouped application including two Type II variations under category C.I.4/C.4*. |
||
| 8 | Grouping of variations affecting unrelated areas of the dossier | Not acceptable for grouping |
|
Example 1: Type II variation under category C.I.4/C.4* to provide 3-year clinical data based on an interim study report from study A with consequential changes to sections 4.8 and 5.1 of the SmPC. In addition, the applicant proposed to provide the final CSR for clinical study B with consequential changes to SmPC section 5.1, and the final CSR for a drug-drug interaction study C with consequential changes to SmPC section 4.5, as well as to take the opportunity to condense the existing text in SmPC section 4.8, to align the annexes with the latest QRD templates and to implement editorial changes in the SmPC. The provision of the interim data from study A and the consequential PI changes constitutes one Type II variation under category C.I.4/C.4*. The provision of the final CSR from study B with a consequential update to section 5.1 of the SmPC constitutes a separate assessment and therefore a separate Type II variation under category C.I.4/C.4* is required. As both studies A and B are clinical (safety and/or efficacy) and affect SmPC section 5.1 it would be meaningful for these variations to be reviewed simultaneously. The final clinical study report for study C concerns a drug-drug interaction study which is not considered consequential or related and will require different expertise for the assessment (clinical pharmacology or non-clinical, depending on the nature of the drug-drug interaction study). Therefore, a separate Type II variation under category C.I.4/C.4* should be submitted. The remaining proposed changes are considered relatively minor and can be included as part of the proposed application without the need for any additional scope i.e. any additional variation. Thus, the applicant should in this case submit one grouped application including 2 Type II variations under category C.I.4/C.4* and one separate stand-alone Type II variation under category C.I.4/C.4*. |
||
| 9 | Grouping of variations in unrelated populations | Not acceptable for grouping |
| Example 1: Data package supportive of 2 different indications e.g. renal cell carcinoma + non-small cell lung cancer. This would not be an acceptable grouping. Separate variations should be submitted. This is because the two indication changes may follow different timelines (i.e. number of Requests for Supplementary Information) and have different outcomes, so that the approval of one indication could be delayed because of the other. | ||
|
Example 2: Provision of the final CSRs for 6 clinical phase 2 and 3 studies undertaken in the same patient population without consequential changes to the PI. The applicant should submit 6 Type II variations under category C.I.13/C.12*. As all 6 studies are clinical and provide safety and/or efficacy data in the same patient population, the scopes are considered related and it is considered meaningful for these variations to be reviewed simultaneously. Thus, the applicant should submit one grouped application including six Type II variations under category C.I.13/C.12*. |
||
* [classification according to variation guideline prior to 15 January 2026 / classification according to variation guideline after 15 January 2026]
Grouped variations applications should contain the elements listed in Annex IV of the Variations Regulation and should be presented in accordance with the appropriate headings and numbering of the EU-CTD format.
The submission requirements as set-out in the PAG sections for the different types of variations will also apply to grouped variations, but the application should be provided as one integrated submission package (i.e. one eCTD sequence) covering all changes resulting from the variations.
Please also refer to “How shall I present my Type II Variation application?”
For a (super-group of) Type IA/ IAIN variation(s) concerning several marketing authorisations, please refer to “When shall I submit my Type IA/IAIN variation(s)?” and Harmonised eCTD Guidance.
References
A procedural/case number will be assigned by the EMA upon receipt of an eCTD application.
The EMA will allocate a ‘high-level’ cross-products procedure/case number shortly before submission. To submit your request, raise a ticket via EMA Service Desk. Please click on “Finance Services”, then the Type of question to be selected is “Request for high-level procedure or ASMF number” followed by sub-option “Super Grouping (Type IA grouping)” and attaching a draft cover letter.
If you do not have an EMA Account, you may create one via the EMA Account Management portal.
Please note that requesting this high level number in advance is mandatory for submissions sent via the eSubmission Gateway or Web Client since this number must be included in eSubmission Gateway XML delivery file User interface.
Grouped variations shall be subject to a worksharing procedure, provided that the same group of variations applies to all medicinal products concerned by the worksharing procedure. However, groups including an extension application are excluded from worksharing.
Based on Articles 7a and 20 of the Variations Regulation when the grouping only consists of
A grouped variation application will be handled and will follow the review procedure of the ‘highest’ variation type in the group.
For example:
When the group follows the timetable of the type II variation, weekly-start timetables may apply to the assessment following the same principles as those applied to the assessment of type II variations.
The assessment timetable may be reduced having regard to the urgency of the matter, particularly for safety issues, or may be extended to 90 days (for agreed grouping of variations or for Type II variations concerning changes or additions to the therapeutic indication).
For more information please refer to the following questions and answers from the post-authorisation guidance for type II variations: ‘Which submission dates (weekly or monthly) are applicable for my type II variation and when shall I submit my application?’ and ‘How shall my type II application be handled (timetable)?’
In case of grouped type IA/ IAIN variations, the Agency will issue a Notification reflecting which variations are accepted or rejected. The MAH shall immediately cease to apply the rejected variation(s) concerned.
For grouping of other types of variations, where not all of the changes applied for can be positively validated, all valid and not valid variations will be clearly listed in the validation letter.
Upon finalisation of the review of the grouped variations, the Agency will issue an opinion/notification reflecting the final outcome of the procedure and in accordance with the ‘highest’ remaining approvable variation in the group. Such opinion/notification will therefore also list any variations which are not considered approvable, unless these have been withdrawn from the group by the holder during the procedure.
For example:
MAH withdraws the extension from the group --> CHMP will adopt a positive opinion on the type II variation only.
MAH does not withdraw the extension from the group --> CHMP will adopt a ‘composite’ opinion reflecting both the negative extension outcome as well as the positive type II.
MAH withdraws the type II from the group --> Agency will issue a positive notification on the type IB variation.
MAH does not withdraw the type II from the group --> CHMP will adopt a ‘composite’ opinion reflecting both the negative type II outcome as well as the positive type IB.
In any case, the assessment report will mention the initial and complete scope of the application (listing all variations initially included in the group) and will clarify the procedural timelines and steps taken during assessment.
For CHMP opinions on extensions and type II variations, the re-examination procedure set-out in Articles 9(2) and 34 (2) of Regulation (EC) No 726/2004 will apply. For further information please refer to the following questions and answers from the post-authorisation guidance for Type II variations ‘Which submission dates (weekly or monthly) are applicable for my Type II variation and when shall I submit my application?’ and ‘ How shall my Type II application be handled (timetable)?’
The post-opinion and decision-making process that will apply to grouped variations will generally be that of the 'highest' type of opinion or notification issued at the end of the procedure.
For information on the post-opinion and decision-making process for type-IA, -IB and -II variations, please refer to 'how and when will the updated annexes become part of the marketing authorisation?' and 'which post-opinion steps apply to my type-II variation and when can I implement the approved changes?'
The decision granting the marketing authorisation following a grouped application will be amended, where necessary, within a year from the date of notification or CHMP opinion for the variation concerned with the exception of the following grouped variations:
Where a super-group of type-IA or -IAIN variations to the terms of several marketing authorisations have been approved, the Commission will update the marketing authorisation with one decision per product concerned, following the yearly decision-making timeframes for type-IA and -IAIN variations.
For information on fees to be paid, applicable fee reductions and payment process, please refer to the Fee Q&As in Annex I, Section 5, on the Fees payable to the European Medicines Agency page.
References