Type-II variations: questions and answers
Human
Regulatory and procedural guidance
This page lists questions that marketing-authorisation holders (MAHs) may have on type-II variations. It provides an overview of the European Medicines Agency's position on issues that are typically addressed in discussions or meetings with MAHs in the post-authorisation phase. Revised topics are marked 'New' or 'Rev.' upon publication.
Updated 15/07/2025:
"11. How shall my Type II application be handled (timetable)?"
"22. Can a non-orphan therapeutic indication be added to an already authorised orphan medicinal product?"
These questions and answers have been produced for guidance only and should be read in conjunction with the rules governing medicinal products in the European Union, volume 2, notice to applicants.
MAHs must in all cases comply with the requirements of Community legislation. Provisions that extend to Iceland, Liechtenstein and Norway by virtue of the European Economic Area agreement are outlined in the relevant sections of the text.
Commission Regulation (EC) No 1234/2008 (the Variations Regulation) defines a major variation of type II as a variation that is not an extension of the marketing authorisation (line extension) and that may have a significant impact on the quality, safety or efficacy of a medicinal product.
The Variations Regulation and the variations guideline set out a list of changes to be considered as type-II variations. In addition, any other change that may have a significant impact on the quality, safety or efficacy of the medicinal product must be submitted as a type-II variation. Refer also to 'when will my variation application be considered a type-II variation or an extension application?' below.
During validation of an 'unforeseen' variation, submitted by the MAH as a type IB variation, the Agency may consider that the proposed variation may have a significant impact on the quality, safety or efficacy of the medicinal product. In such case, the MAH will be requested to revise and supplement its variation application so that the requirements for a type II variation application are met (see “How shall my type IB variations be handled (timetable)?”).
References
There is generally no requirement to notify the Agency in advance of an upcoming submission of a type II variation. For type II variations entailing additions of new therapeutic indication(s) or modification of already approved one(s) under scope C.I.6, due to the substantial amount of data expected, the assessment timeframe is typically longer (see also question “How shall my Type II application be handled (timetable)”) and significant assessment resources need to be committed by the Rapporteur and usually also from the Co-Rapporteur (see also question “Is the Co-Rapporteur involved in Type II Variations”). For this reason, MAHs are requested to give an advance notice of their intention to submit an extension of indication or other changes to the authorised therapeutic indication ideally 6 months in advance of the planned submission. This can be achieved by means of an email to the Product Lead, , the Rapporteur, Co-Rapporteur and, if applicable, PRAC Rapporteur, summarising the scope of the intended application and specifying the target submission date. The information will be used for planning purposes by the Agency and the Rapporteurs’ assessment teams.
The CHMP leads the assessment of most type II variations and always adopts the final Opinion for type II variations.
However, in case of type II variations concerning clinical safety to update the product information and/or the Risk Management Plan upon request by the PRAC, as a follow-up to a previous PSUR procedure or following a previous PRAC assessment of a signal, the PRAC will take the lead in the assessment of the variation.
It should be noted that the CHMP will lead the assessment of a post-PSUR variation where the scope is related to other aspects of the dossier e.g. non-clinical data, clinical pharmacology and/or clinical efficacy. In addition, the PRAC will lead in the assessment of type II variations:
- Specifically intended to update the RMP;
- Or providing final results of non-interventional post-authorisation safety studies (PASS).
The latter refers to both imposed (PASS category 1 and 2 in the RMP) and requested non-interventional studies (PASS category 3 in the RMP), and regardless of whether or not consequential changes to the product information are proposed.
It should be noted that final results of imposed non-interventional studies are expected to be submitted under the Art 107q of Directive 2001/83/EC procedure (please also refer to guidance on post-authorisation safety studies). Please also refer to “How should non-clinical and/or clinical study reports be provided?” for further guidance on the submission of PASS results.
Whether the CHMP or the PRAC will take the lead in the assessment of the variation will be decided at the time of the validation and communicated to the applicant through the assessment timetable.
It should be noted that the CAT, instead of the CHMP, will take the lead in the assessment of type II variations for advanced therapy medicinal products (ATMPs), unless these are PRAC-led. The CAT will adopt a draft Opinion for all type II variations for ATMPs, including for PRAC-led ones, with the CHMP adopting the final Opinion.
The CHMP (or CAT for ATMPs) Co-Rapporteur is normally not involved in the assessment of a type II variation application concerning quality, non-clinical and clinical including product information changes and RMP updates.
However, the involvement of the CHMP Co-Rapporteur is in most cases deemed necessary for the assessment of a new therapeutic indication or modification of an approved indication (i.e. type II variations under category C.I.6.a).
The MAH should therefore inform the Agency (Product Lead) of an upcoming type II application for a new indication ideally 6 months before submission, so that the CHMP is informed of the future submission and can agree on the Co-Rapporteur’s involvement.
At the time of validation the Agency will inform the MAH of the involvement of the CHMP Co-Rapporteur through the assessment timetable which will refer to the relevant assessment reports expected from the Co-Rapporteur as appropriate.
Regarding the submission of a type II variation application to the (Co-) Rapporteurs, please see also question “How and to whom shall I submit my Type II Variation application” below
As explained in the question “Which Committee will take the lead in the assessment of a type II variation?” above, the PRAC Rapporteur is involved in and performs the primary assessment of PRAC-led variations.
In addition, the PRAC Rapporteur will systematically be involved in the assessment of all CHMP-led type II variations that include an updated RMP for the purposes of assessing the proposed RMP changes.
The CHMP may also on a case-by-case basis involve the PRAC Rapporteur in the assessment of other type II variations during the assessment procedure, e.g. variations involving a Direct Healthcare Professional Communication, following a CHMP request for formal PRAC advice, i.e. input from PRAC on particular safety issues and in response to specific questions raised by the CHMP.
Marketing authorisation holders may choose to group the submission of several Type II variations for the same product into one application, provided that this corresponds to one of the cases listed in Annex III of the Variations Regulation or when this has been agreed upfront with the Agency.
It is also possible for a marketing authorisation holder to group a Type II variation with other variation(s) (e.g. Type IB or IA variations) or extension applications. Such grouped submissions will follow the assessment timetable of the highest variation in the group. Please also refer to What types of variations can be grouped?”
Where the same Type II variation(s) affect(s) one or more marketing authorisations from the same holder, the marketing authorisation shall submit these variations as one application for ‘worksharing’. Please also refer to “What is worksharing and what types of variations can be subject to worksharing?”
References
A type II variation application should contain the elements listed in Annex IV of the Variations Regulation and should be presented in accordance with the appropriate headings and numbering of the EU-CTD format.
In addition, the MAHs are expected to complete the relevant validation checklist (Validation checklist for Type II (non) clinical variations or Validation checklist for Type II quality variations) and submit it as a word document (as part of the working documents) in Module 1 as an Annex. The checklist will help MAHs to ensure that their type II variations are complete and in compliance with legal and regulatory requirements, leading to a smoother validation.
The Commission ‘Variations Guidelines’ further specifies which elements should be included in a Type II variation application. More specifically, a type II variation application should contain the following elements:
Module 1
Module 2
In order to facilitate the assessment, the relevant Module 2 update(s) or addendum(s) should also be provided as Word document in the ‘working documents’ outside the eCTD structure (see below).
Modules 3, 4 and 5
The applicant can cross refer to information already included in the same dossier by using hyperlinks in modules 3, 4 and/or 5 rather than re-submitting the data again.
Working documents outside the eCTD structure:
Additional Word formats of certain documents are required to facilitate the assessment i.e. ‘tracked changes’ versions for SmPCs, RMPs or other documents specified by the Agency such as relevant Module 2 update(s) or addendum(s). These should be provided in the separate folder ‘XXXX-working documents’. Further details can be found in the Harmonised technical Guidance for eCTD Submissions in the EU. It is generally not necessary to include the RMP annexes in the ‘working document’ version (unless annexes are being revised).
The above requirements also apply to the submission of the validation checklist (see above) and the responses to Request(s) for Supplementary Information.
See also “How should I present a grouped-variation application?” and “How should I present a variation application under worksharing?”
It should be noted that the responsibility for the quality of the submitted documentation lies with the MAH and is crucial to the overall process.
For queries relating to the presentation of the application, please contact the Agency (allocated Product Lead).
References
The MAHs are expected to complete the relevant validation checklist:
Validation checklist for Type II (non) clinical variationsor
Validation checklist for Type II quality variationsand submit it as a word document (as part of the working documents) in Module 1 as an Annex. The checklist will help MAHs to ensure that their type II variations are complete and in compliance with legal and regulatory requirements, leading to a smoother validation.
In addition to the requirements foreseen in the question above, the following considerations specifically apply to applications concerning a new or a modified indication (please refer to question ‘What is considered a new or modified therapeutic indication?’)
Please also refer to Q&As on ‘20. What aspects should I consider at time of submission of a type II variation if there are orphan medicinal products designated or authorised for a condition related to my proposed therapeutic indication?’, ‘21. Do I need to confirm the maintenance of my orphan designation when applying for a type II variation?’, ‘22. Can a non-orphan therapeutic indication be added to an already authorised orphan medicinal product?’ and ‘24. Do I need to address any paediatric requirements in my type II variation application?’.
Working documents outside the eCTD structure:
Additional Word formats of certain documents are required to facilitate the scientific assessment by the relevant scientific bodies i.e. ‘tracked changes’ versions for SmPCs, RMPs or other documents specified by the Agency such as relevant Module 2 update(s) or addendum(s) and the summary of the main efficacy results. These should be provided in the separate folder ‘XXXX-working documents’. Further details can be found in the Harmonised technical Guidance for eCTD Submissions in the EU. It is generally not necessary to include the RMP annexes in the ‘working document’ version (unless annexes are being revised).
The above requirements also apply to the submission of the validation checklist (see above) and the responses to Request(s) for Supplementary Information.
Please also refer to the following questions which address paediatric related aspects ‘24. Do I need to address any paediatric requirements in my type II variation application?’ and ‘What is considered a new or modified therapeutic indication?’.
References
Information is available on ‘Submitting a post-authorisation application'.
The assessment timetable and hence the submission deadline applicable to a type II variation application depends on the committees involved in the assessment, the amount of assessment needed and whether the CHMP Opinion will be followed by an amendment of the Commission Decision granting the Marketing Authorisation within two months.
There are two types of submission deadlines and consequently procedure start dates: monthly and weekly once.
Weekly starts are applicable to the majority of the type II variation applications received by the Agency. The following minority of type II variations applications follow a monthly start date:
Specific monthly start dates apply for variations involving the PRAC. Opinions for monthly start variations requiring Commission Decision within two months from CHMP Opinion (including extensions of indication) are adopted during the week of the CHMP plenary meeting. Opinions for monthly start variations involving the PRAC and not requiring Commission Decision within two months are adopted during the week of the PRAC plenary meeting. Opinions for weekly start variations are adopted independently of the committee plenary meetings.
For variations following the weekly start, the Agency may need to amend the timetable if during the procedure the need for discussion at plenary / involvement of other committees (e.g. PRAC), working parties (i.e. BWP) or for immediate EC decision arise.
In case there is uncertainty before submission as to which timetables and submission deadlines are to be followed, MAHs can request the advice of the Agency by contacting the allocated Product Lead. The Agency will inform the MAH of the applicable timetable in the validation confirmation e-mail. For more information see also question ‘How shall my Type II application be handled (timetable)?’.
For both weekly-start and monthly-start assessment timetables, the MAH should submit their application at the latest by the recommended submission dates published on the Agency’s website (Please refer to “Human Medicines – Procedural Timetables / Submission dates”).
MAHs are reminded of their legal obligation to submit forthwith any information that becomes available which might entail the variation of the MA.
Where the CHMP requests the submission of a variation following the assessment of a post-authorisation measure (PAM), Specific Obligation (SO) or signal, MAHs must submit the corresponding variation application within the requested timeframe.
Variation applications reflecting the outcome of an Urgent Safety Restriction (USR) shall be submitted immediately and in any case no later than 15 days after the initiation of the USR to the Agency. This applies to USRs initiated by the MAH or imposed by the European Commission.
Implementation of agreed wording changes following the above mentioned procedures for which no additional data are submitted by the MAH will follow a Type IB variation procedure.
References
Upon receipt of a technically valid application, the Validation Team (Non-clinical/Clinical/RMP variations) or Quality Specialist (Quality variations) will perform the validation of the application content. Supplementary information may be requested in order for the validation to be finalised and the procedure will commence at the next available start date after resolution of issues identified during validation. The Agency will inform the MAH of the outcome of the validation and timetable (TT).
Assessment of Type II variations following a 60-day TT may either follow a weekly or a monthly start date, depending whether the variation needs to be aligned with the CHMP plenary meeting periodicity (See also question “When shall I submit my application?” above).
Extensions of indication on a 90-day TT always follow the monthly start TT. They are discussed during the CHMP plenary meeting and require a Commission Decision (CD) to be adopted within two months from CHMP Opinion.
Type II variation procedures following a 30-day TT (e.g. urgent safety issues) will in principle follow the monthly start TT. They are likely to be discussed during the CHMP plenary meeting and require the adoption of a Commission Decision (CD) within two months from CHMP Opinion.
For variations following a weekly-start TT, the opinion or request for supplementary information (RSI) will be adopted by the CHMP independently of the plenary meetings. The MAH can also provide their responses to an RSI during the procedure in line with the weekly re-start dates.
Variations following a 60 day TT (= standard TT):
Condition:
Variations assessed by the CHMP only or variations involving the PRAC (refer to question 'Is the PRAC Rapporteur involved in Type II Variations?') not requiring CD within two months from CHMP Opinion:
| Day | Action | |
|---|---|---|
| Day 1 | Start of evaluation | |
| Day 36 | Receipt of CHMP# Rapporteur's Assessment Report | |
| Day 43^ | Receipt of PRAC Rapporteur's Assessment Report | |
| Day 47^ | Comments by other PRAC members | |
| Day 50 | Comments by other CHMP members | |
| Day 51^ | Receipt of PRAC Rapporteur's updated Assessment Report* | |
| Day 53 | Receipt of CHMP# Rapporteur's updated Assessment Report* | |
| Day 58^ | PRAC outcome | |
| Day 60 |
Adoption of the CHMP Opinion [or Request for supplementary information] |
|
*Updated assessment reports are optional, depending on comments received by other committee members.
#There is(are) no CHMP Rapporteur's assessment report(s) in case of PRAC-led variations.
^Steps not applicable for CHMP-only variations.
Variations assessed by PRAC (refer to question 'Is the PRAC Rapporteur involved in Type II Variations?') and CHMP requiring CD within two months from CHMP Opinion:
| Day | Action | |
|---|---|---|
| Day 1 | Start of evaluation | |
| Day 30 | Receipt of CHMP Rapporteur's Assessment Report | |
| Day 33 | Receipt of PRAC Rapporteur's Assessment Report | |
| Day 38 | Comments by other PRAC members | |
| Day 39 | Receipt of PRAC Rapporteur's updated Assessment Report* | |
| Day 46 | PRAC outcome | |
| Day 50 | Comments by other CHMP members | |
| Day 53 | Receipt of CHMP Rapporteur's updated Assessment Report* | |
| Day 60 |
Adoption of the CHMP Opinion [or Request for supplementary information] |
|
*Updated assessment reports are optional, depending on comments received by other committee members.
Variations following a 30 day TT:
Condition:
Variations assessed by the CHMP only or variations involving the PRAC (refer to question 'Is the PRAC Rapporteur involved in Type II Variations?') not requiring CD within two months from CHMP Opinion:
| Day | Action |
|---|---|
| Day 1 | Start of evaluation |
| Day 15 | Receipt of CHMP# Rapporteur's Assessment Report+ |
| Day 17^ | Receipt of PRAC Rapporteur's Assessment Report |
| Day 20^ | Comments by other PRAC members |
| Day 20 | Comments by other CHMP Members+ |
| Day 21^ | Receipt of PRAC Rapporteur's updated Assessment Report* |
| Day 23 | Receipt of CHMP# and PRAC Rapporteur's updated Assessment Report*+ |
| Day 28^ | PRAC outcome |
| Day 30 |
Adoption of the CHMP Opinion [or Request for supplementary information] |
*Updated assessment reports are optional, depending on comments received by other committee members.
#There is(are) no CHMP Rapporteur's assessment report(s) in case of PRAC-led variations.
^ Steps not applicable for CHMP-only variations.
+ For the initial submission assessment of CHMP-only variations following a weekly start 30-day TT. the CHMP assessment report, CHMP members comments and CHMP updated assessment report is foreseen at Day 20, 22 and 24 respectively.
Variations assessed by PRAC (refer to question 'Is the PRAC Rapporteur involved in Type II Variations?') and CHMP requiring Commission Decision within two months from CHMP Opinion:
| Day | Action |
|---|---|
| Day 1 | Start of evaluation |
| Day 6 | Receipt of PRAC Rapporteur's Assessment Report |
| Day 8 | Comments by other PRAC Members |
| Day 9 | Receipt of PRAC Rapporteur's updated Assessment Report* |
| Day 15 | Receipt of CHMP Rapporteur's Assessment Report |
| Day 16 | PRAC outcome |
| Day 20 | Comments by other CHMP Members |
| Day 23 | Receipt of CHMP Rapporteur's updated Assessment Report* |
| Day 30 |
Adoption of the CHMP Opinion [or Request for supplementary information] |
*Updated assessment reports are optional, depending on comments received by other committee members.
In exceptional cases, this timetable could be further shortened.
Variations following a 90 day TT:
Condition:
| Day | Action |
|---|---|
| Day 1 | Start of evaluation |
| Day 56 | Receipt of and CHMP (Co-) Rapporteur's Assessment Report |
| Day 63^ | Receipt of PRAC Rapporteur's Assessment Report |
| Day 68 | Comments by other PRAC members^ |
| Day 69^ | Receipt of PRAC Rapporteur's updated Assessment Report |
| Day 76^ | PRAC outcome |
| Day 80 | Comments by other CHMP members |
| Day 83 | Receipt of CHMP Rapporteurs' Joint Assessment Report |
| Day 90 |
Adoption of the CHMP Opinion [or Request for supplementary info] |
^The PRAC is normally involved in the assessment of Type II variation applications following the 90-day TT as an (updated) RMP is expected to be submitted as part of the application. Absence of an RMP update should be justified at the time of submission.
In case issues which prevent the adoption of an Opinion are identified at D90, the CHMP will adopt an RSI together with a deadline for submission of the requested data by the MAH and a TT for the assessment of the MAH’s responses. The MAH will receive the adopted TT embedded within the RSI. The clock will be stopped until the receipt of the MAH’s response to the RSI.
Responses to the RSI must be sent to the Agency, as per the instructions included in the “Submitting post-authorisation application” section of the Post-authorisation guidance.
Clock-stop scenarios:
For clock-stops longer than 1 month (clock-stop extension), the MAH should send a justified written request to the Agency for agreement by the Rapporteur(s) and the corresponding Committee(s):
Assessment of responses
The Committee assessment of the MAH’s responses will take up to 30 or 60 days depending on the complexity and amount of data provided by the MAH. Upon receipt of the responses from the MAH, the procedure will be re-started following a weekly-start, alternative monthly or monthly-start timetable according to the same principles as the ones applied at the initial start of procedure.
Oral explanation
An oral explanation in front of the relevant Committee can be held at the request of the Committee or the MAH, where appropriate.
References
When two or several stand-alone type II variation applications are being submitted and/or assessed in parallel the following general principles apply:
In order to simplify the handling of different versions of the Product Information, submissions affecting the Product Information should be whenever possible combined in a grouped variation application, if allowed by grouping rules. Please also refer to “What groups of variations would be considered acceptable?”.
Once a CHMP opinion has been adopted for a type II variation, or a Commission Decision has been granted in case an immediate EC Decision applies, the approved Product Information can be used as baseline for the Product Information of any subsequent variation(s). The consolidation can be done at the time of any procedural milestone of the subsequent variation(s) e.g. as part of the MAH's responses to a request for supplementary information, but in any case at the latest before the adoption of the CHMP opinion.
Once included, the already approved changes related to a previous variation should appear as clean text in both the clean and highlighted versions of the Product Information for subsequent variation(s). It should be noted that only the new proposed changes related to the subsequent variation should continue to be highlighted in tracked changes during that procedure.
Upon adoption of the CHMP opinion, the Agency will inform the MAH within 15 days as to whether the CHMP opinion is favourable or unfavourable (including the grounds for the unfavourable outcome), as well as whether the Commission Decision granting the marketing authorisation requires any amendments.
Where the outcome of the procedure is favourable and the Commission Decision granting the Marketing Authorisation requires amendments, the Agency will inform the Commission accordingly.
Re-examination
Art. 9(2) of Regulation (EC) No 726/2004, also applies to CHMP Opinions adopted for Type II variation applications. This means that the MAH may give written notice to the Agency/CHMP that he wishes to request a re-examination within 15 days of receipt of the opinion (after which, if he does not appeal, the opinion shall be considered as final). The grounds for the re-examination request must be forwarded to the Agency within 60 days of receipt of the opinion. In case the MAH requests that the committee consults a Scientific Advisory Group (SAG) in connection with the re-examination, the applicant should inform the CHMP as soon as possible of this request.
The CHMP will appoint different CHMP (Co-) Rapporteurs, to co-ordinate the re-examination procedure. In case a PRAC Rapporteur is deemed necessary, he/she will be appointed. Within 60 days from the receipt of the grounds for re-examination, the CHMP will consider whether its opinion is to be revised. If considered necessary, an oral explanation can be held within this 60-days timeframe.
EMA charges a fee for a re-examination of an opinion. For more information, please refer to the Fee Q&As in Annex IV, Section 4, on the Fees payable to the European Medicines Agency.
Linguistic review
Where the product information is affected, a linguistic review of the Product Information changes will be performed. The linguistic review will start 5 days after the CHMP plenary meeting following the adoption of the CHMP opinion on the variation. The monthly linguistic review will cover all procedures affecting the annexes concluded since the latest linguistic review i.e. all variations adopted in line with the ‘weekly-start’ timetables as well as those following the ‘monthly’ timetables that have had an opinion adopted at the CHMP plenary meeting in the same month will be included. The EPAR update will also consolidate all procedures concluded since the latest EPAR update.
In the event that the only change to the Product Information concerns deletion of text or a change to numerical characters e.g. shelf life of a finished product, no post-opinion linguistic review would be necessary.
In all cases, the amended Product Information in all languages should be provided by the MAH by the date specified in the translation timetable which is provided with the CHMP opinion.
Decision-Making Process
Upon receipt of a favourable CHMP opinion which requires amendments to the decision granting the marketing authorisation, the Commission shall amend the marketing authorisation to reflect the variation within 2 months, for the variations listed under Article 23(1a)(a) or within one year for the other type II variations.
Article 23(1a)(a) provides for a two month timeframe for amending the Commission decision granting the marketing authorisation for the following variations:
All the other type II variations will follow a yearly timeframe for update of the respective Commission decision.
Timeline for variations - post opinion
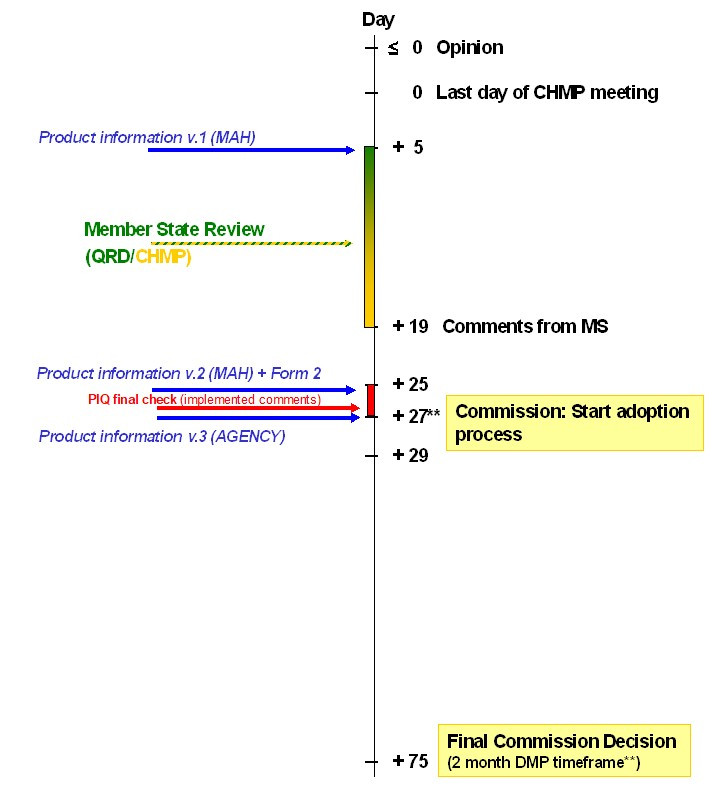
**applicable only to Type II variations listed under Art. 23.1a(a) of Commission Regulation (EC) No 1234/2008
Where a group of variations to the terms of one marketing authorisation submitted as part of one variation have been approved, the Commission will update the marketing authorisation with one single decision to cover all the approved variations.
Implementation
Type II variations listed in Article 23(1a)(a) may only be implemented once the Commission has amended the marketing authorisation and has notified the MAH accordingly. Variations related to safety issues, including urgent safety restrictions, must be implemented without delay and/or within a timeframe agreed by the MAH and the Agency.
Type II variations which do not require any amendment of the marketing authorisation or which follow a yearly update of the respective Commission Decision can be implemented once the MAH has been informed of the favourable outcome by the Agency. However, it is expected that where the variation includes changes to the product information, the MAH waits for the finalisation of the linguistic review process by the Agency before implementing the variation, as appropriately checked translations are considered essential for a correct implementation of the variation.
The agreed change(s) should be included in the product information annexes of any subsequent regulatory procedure.
See also question “How should parallel type II variations that affect the Product Information (PI) be handled?” above.
Date of revision of the text
The date of revision of the text to be included in section 10 of the SmPC and corresponding section of the package leaflet for variations affecting the product information should be as follows:
This date corresponds to the date of EC decision or CHMP opinion when that specific annex was affected.
References
For information on fees to be paid, applicable fee reductions and payment process, please refer to the Fee Q&As in Annex I, Section 5, on the Fees payable to the European Medicines Agency.
References
For information concerning submission of mock-ups and specimens in the framework of post-authorisation procedures, please refer to the document 'Checking process of mock-ups and specimens of outer/immediate labelling and package leaflet of human medicinal products in the centralised procedure, 3.4 Other post-authorisation procedures
References
In case the type II Variation affects the SmPC, Annex II, labelling and/or package leaflet, the revised product information Annexes must be submitted as follows:
At submission
During the procedure
In addition, during the latter stages of the procedure there is often a need for fast informal exchanges between the MAH and the Rapporteur in preparation of the final CHMP opinion. During this process the MAH can provide any revised versions of the product information as well as comments/justifications by Eudralink/email in Word format. These product information versions are considered 'working documents' only and there is consequently no need to submit these updated product information proposals as part of a formal eCTD sequence (unless part of formal responses to a CHMP request for supplementary information).
See also question “How should parallel type II variations that affect the PI be handled?” above.
At CHMP Opinion (Day 0)
After CHMP Opinion (Day +5, for all variations with an Opinion that month – both those on a weekly-start timetable and those on a monthly-start timetable, this is 5 days after the CHMP plenary meeting following the adoption of the CHMP opinion)
After Linguistic check (Day +25, for all variations that month – both those on a weekly-start timetable and those on a monthly-start timetable, this is 25 days after the CHMP plenary meeting following the adoption of the CHMP opinion)
The final adopted annexes should always be provided post opinion as part of an eCTD closing sequence within 15 days of the Commission Decision (if there is one) or within 2 weeks after the finalisation of the linguistic review process (if this is not followed by a Commission Decision).
Overview:
| Day | Lang.* | Post-opinion linguistic review Timetable |
| 0 | EN |
Electronically Word format (highlighted) |
| +5 | All EEA |
Electronically Word format (highlighted) |
| +25 | All EEA |
Electronically Word format (highlighted) PDF format (clean) |
* = complete set of Annexes i.e. Annex I, II, IIIA and IIIB submitted as one document per language
The 'complete set of Annexes' includes Annex, I, II, IIIA and IIIB i.e. all SmPC, labelling and package leaflet texts for all strengths and pharmaceutical forms of the product concerned, as well as Annex II.
The complete set of Annexes must be presented sequentially (i.e. Annex I, II, IIIA, IIIB) as one document for each official EU language. Page numbering should start with "1" (bottom, centre) on the title page of Annex I. The 'QRD Convention' published on the Agency's website should be followed. When submitting the full set of Annexes in PDF format, this should be accompanied by the completed which provides User guide on how to generate PDF versions of the product information - human on how to correctly prepare the PDF versions.
The electronic copy of all languages should be provided as part of the variation application on the Gateway / Web Client package. Highlighted changes should be indicated via 'Tools – Track changes'. Clean versions should have all changes 'accepted'.
Icelandic and Norwegian language versions must always be included.
At the time of the submission and throughout the procedure, the annexes provided should only reflect as highlighted text the changes introduced by the specific variation concerned. However, following adoption of the CHMP opinion it may be necessary to consolidate the adopted annexes for separate variations running in parallel, i.e. when these conclude concurrently. In that case the linguistic review will be undertaken based on the consolidated version which should reflect as highlighted text all changes for the parallel variations adopted by the CHMP at that plenary meeting and including variations adopted earlier during the month in line with the weekly-start timetable.
The section “present/proposed” in the application form should clearly list all changes proposed to the English annexes. Any minor linguistic amendments introduced for other languages should be provided as a separate document attached to the application form.
In such cases and in cases where any other ongoing procedures may affect the product information annexes, the MAH is advised to contact the Agency in advance of submission or finalisation of the procedure(s) concerned.
For those variations which affect the Annex A (e.g. introduction of a new presentation), the following principles apply:
Upon adoption of the opinion, the Agency will prepare and send to the MAH the revised English Annex A reflecting the new/amended presentation.
After CHMP Opinion (Day +5, for variations on a weekly-start timetable, this is 5 days after the CHMP plenary meeting following the adoption of the CHMP opinion), the MAH provides the Agency with the electronic versions of the complete set of annexes in all languages as well as the translations of the revised Annex A as a separate word document.
Please be reminded that in accordance with Union data protection requirements, no personal data should be included in the annotated PIs. This applies to the English version submitted at the time of opinion, the draft translation versions of the PI in all the languages submitted at D+5 as well as the final translations submitted at D+25. The annotated product information files must include the statement containing the procedure number(s) and may be published on the EMA website as part of the product EPAR page. Please submit annotated PIs in an anonymised format (i.e. names of the reviewers removed from the track-changes). If you do not wish to do so, please ensure that the individuals whose data is included consented to its sharing with EMA, the publication on the EMA website and its further sharing by EMA with third parties such as other marketing authorisation applicants, marketing authorisation holders and National Competent Authorities, as relevant. EMA expressly disclaims any liability or accountability for the presence of unnecessary personal data in the annotated PI submitted by the marketing authorisation holder.
Any changes in the number of units of medicinal product or medical device being an integral part of the medicinal product (e.g. prefilled syringes) will trigger a different EU number.
Differentiation should be made between the addition of a presentation where the two presentations will co-exist on the market on a long-term basis versus a replacement of a presentation where the new presentation will replace the previous one (it is expected that for a certain period of time, the two presentations will co-exist on the market until the stock of the previous presentation runs out).
In principle, a replacement of one presentation by another presentation does not trigger a new EU number, unless the number of units of medicinal product or medical device being an integral part of the medicinal product (e.g. prefilled syringes) is changed.
Examples of changes in presentations for replacement, not triggering a new EU number (this is not an exhaustive list):
In case of addition, as the presentations will co-exist on the market, two packs with different contents cannot be covered by the same EU number and will be considered as different presentations.
Changes in the number of any unit (not restricted to the medicinal product) or changes in the specifications of any unit (not restricted to the medicinal product) contained in the pack will trigger a new EU number.
Examples of changes that will trigger new EU numbers (this is not an exhaustive list):
If you have any questions on any upcoming submission, please contact the allocated Product Lead.
At the time of the adoption of a CHMP opinion for a type II variation which includes additional presentation(s), the Agency will assign the new EU sub-numbers and include them in the revised Annex A of the medicinal product, which will be transmitted to the marketing authorisation holder together with the CHMP Opinion and respective annexes.
The marketing authorisation holder should include the newly assigned numbers in all language versions of the Annex A and in all applicable sections of the product information, which are submitted following the CHMP opinion for linguistic review.
The meeting highlights following each CHMP meeting give information on opinions in relation to new indications, changes to an existing indication and the addition, change or removal of a contraindication. This will include the name of the product, the name of the MAH, the indication(s). Where applicable, the CHMP gives also an update on safety information.
Please refer also to “What we publish on medicines and when?”.
References
Type II variations for a new indication, which is the same as the indication of an authorised Orphan Medicinal Product, should include relevant information in Module 1.7 of the application, based on the following considerations:
In accordance with Article 8.1 of Regulation (EC) No 141/2000, where a marketing authorisation in respect of an orphan medicinal product has been granted in all Members States, the Union and the Member States shall not, for a period of 10 years, accept another application for marketing authorisation, or grant a marketing authorisation or accept an application to extend an existing marketing authorisation, for the same therapeutic indication, in respect of a similar medicinal product.
Where a designated orphan medicinal product has been authorised for the condition which covers the proposed therapeutic indication being applied for, and a period of market exclusivity is in force, the MAH must submit a report in module 1.7.1 addressing the possible “similarity” with the authorised orphan medicinal product (even if the concerned product does not have orphan designation).
The assessment of similarity between two medicinal products takes into consideration the following criteria:
The critical report provided in Module 1.7.1 should address the possible similarity between the proposed new medicinal product and the authorised orphan medicinal products for each of these criteria.
If significant differences exist within one or more of these criteria, the two products will not be considered as similar. These criteria are explained in the Guideline on aspects of the application of Article 8(1) and 8(3) of Regulation (EC) No 141/2000: Assessing similarity of If significant differences exist within one or more of these criteria, the two products will not be considered as similar. Commission Regulation (EC) No 847/2000 provides additional specific considerations for the definition of similar active substance applicable to chemical, biological and advanced therapy medicinal products.
If the medicinal product is deemed to be “similar” to an authorised orphan medicinal product, the MAH must furthermore provide justification in module 1.7.2 that one of the derogations laid down in Article 8.3, paragraphs (a) to (c) of the same Regulation applies, namely:
(a) the holder of the marketing authorisation for the original orphan medicinal product has given his consent to the second applicant, or
(b) the holder of the marketing authorisation for the original orphan medicinal product is unable to supply sufficient quantities of the medicinal product, or
(c) the second applicant can establish in the application that the second medicinal product, although similar to the orphan medicinal product already authorised, is safer, more effective or otherwise clinically superior.
The assessment of similarity is conducted in parallel to the evaluation of the variation application and follows the same timetable. The assessment includes the consultation of the Quality Working Party or the Biologicals Working Party for the aspects concerning the similarity of the molecular structures of the products.
Even if the variation does not concern an orphan designated product, all MAHs should still check whether their claimed new indication would potentially overlap with the indication of authorised orphan medicinal products, as listed on the Commission Website in the “Community register” of designated orphan medicinal products and include the relevant documentation in their variation application as set-out above.
References
If the product has been designated as orphan and the application concerns a new therapeutic indication or a modification of an existing one, in order to ensure that the Marketing Authorisation only covers indications that fulfil the orphan designation criteria foreseen in Art 3 of Regulation (EC) No 141/2000, a COMP review may be required as following:
To support this process, the MAH/sponsor is requested to provide at the time of submission of the variation either a justification that the variation does not raise doubts on the fulfilment of the orphan criteria or a maintenance report to justify that the orphan criteria are still met. The justification/ maintenance report should be should be submitted via the IRIS Platform.
Further to the COMP preliminary discussion based on the sponsor’s justification/ maintenance report, a formal review process of the maintenance of the orphan designation for the applied indication will be triggered if this raises justified and serious doubts on the maintenance of the orphan designation. In this case, if previously only a justification was submitted, the MAH/sponsor will be requested to provide a maintenance report. The procedure for assessment will follow the usual procedure, as described in .
For the purpose of defining what is a new therapeutic indication or a modification of an existing one for the COMP review for post-authorisation extensions of indications,the Guideline on the elements required to support the significant clinical benefit in comparison to existing therapies of a new therapeutic indication in order to benefit from an extended (11-year) marketing protection should be followed.
In case of any doubts, the Agency encourages applicants to contact the Orphan Medicines Office in advance of a planned submission in order to clarify orphan requirements. Please submit your message via Send a question to the European Medicines Agency.
Further information can be found on the dedicated EMA website on orphan designation.
References
As provided for in Article 7(3) of the Orphan Regulation, it is not possible to combine within the same marketing authorisation orphan and non-orphan indications. In case you wish to extend the therapeutic indications of your orphan medicinal product to include additional non-orphan therapeutic indications, you will have to consider the following regulatory options:
If the orphan designation is not yet withdrawn at time of submission, the marketing authorisation holder should undertake in their cover letter to request the withdrawal the orphan designation from the Union register no later than 2 days after the receipt of the CHMP opinion.
Based on this commitment, the Agency will validate the variation / MA extension application pertaining to a non-orphan indication. If the MAH has not requested the withdrawal of the Orphan designation within the said deadline, nor requested re-examination in accordance with Article 16(4) of Commission Regulation (EC) No. 1234/2008, the validation of application will become automatically null and void with retroactive effect.
Sponsors should use EMA’s IRIS system to submit the request to remove the orphan designation from the Union Register. To request removal, the sponsor should:
Upon receiving the submission, EMA will forward the request to the European Commission, who will notify the removal to the sponsor and update the Union Register accordingly.
References
According to Articles 14-a and 14(8) of the Regulation (EC) No 726/2004, a marketing authorisation can be granted in certain situations based on less comprehensive data than normally required, i.e. a conditional marketing authorisation or marketing authorisation under exceptional circumstances, respectively.
Granting these types of authorisation is only foreseen in the context of an application for an initial marketing authorisation. Therefore, when a “standard”/“full” marketing authorisation has been already granted, it is not possible to subsequently change this authorisation into a conditional marketing authorisation or a marketing authorisation under exceptional circumstances. In such case, introduction of a new indication within the same marketing authorisation will have to comply with the standard data requirements. Alternatively, submission of a separate marketing authorisation (either conditional of under exceptional circumstances) may be required, taking into account also provisions concerning multiple applications.
Nevertheless, if a product already has a conditional marketing authorisation, it is possible to modify (including extend) the indication and related specific obligations, provided that any modifications that are based on less comprehensive data comply with the requirements for a conditional marketing authorisation. These requirements are set out in Article 14-a of Regulation (EC) No 726/2004 and in Commission Regulation (EC) No 507/2006 and further elaborated in the respective “Guideline on the scientific application and the practical arrangements necessary to implement Commission Regulation (EC) No 507/2006 on the conditional marketing authorisation for medicinal products for human use falling...”.
Similarly, if a product has a marketing authorisation under exceptional circumstances, it is possible to modify (including extend) the indication and related specific obligations, provided that any modifications based on less comprehensive data comply with the requirements for a marketing authorisation under exceptional circumstances. These requirements are set out in Article 14 (8) of the Regulation (EC) No 726/2004 and in Part II of Annex I of Directive 2001/83/EC, and further elaborated in the respective “Guideline on procedures for the granting of a marketing authorisation under exceptional circumstances, pursuant to article 14 (8) of Regulation (EC) No 726/2004”.
References
Regulation (EC) No 1901/2006, as amended (the 'Paediatric Regulation') lays down obligations, rewards and incentives for the development and placing on the market of medicines for use in children. The Paediatric Regulation places some obligations for the applicant when developing a new medicinal product as well as new uses of an authorised product, in order to ensure that medicines to treat children are subject to ethical research of high quality and are appropriately authorised for use in children, and to improve collection of information on the use of medicines in the various subsets of the paediatric population. The paediatric population is defined as the population between birth and the age of 18 years (meaning up to but not including 18-years).
As set out in Article 8 of the Paediatric Regulation, applications for new indication(s), new pharmaceutical form(s) and/or new route(s) of administration concerning an authorised medicinal product protected either by a supplementary protection certificate or by a patent which qualifies for the granting of such a certificate must include one of the following documents/data in order to be considered 'valid':
This means that the application will have to include the PIP decision but also the results in accordance with the agreed PIP.
This means that the application will have to include the PIP decision including the deferral granted and if applicable, any completed studies.
This requirement applies irrespective of the type of application submitted for such a change(s) i.e. variation or extension (or new marketing authorisation application) and irrespective of whether the change is related to adult or paediatric use.
To define what is a ‘new indication’ for the purpose of the application of Article 8, please see 'Paediatric investigation plans: questions and answers', under section 'Articles 7 and 8: Definitions'.
Where results of PIP studies do not support a paediatric indication, the corresponding proposal for amending the Product Information may be submitted as part of a variation C.I.4 as per the guideline on the details of the various categories of variations – 'Variations related to significant modifications to the SmPC'.Applicants are requested to mention in the application form of the variation including the paediatric results and in the cover letter the following statement in the section 'Precise scope and background for change': 'Submission of paediatric study results performed in compliance with a paediatric investigation plan which do not support a paediatric indication'.
Applicants should include in the clinical overview a rationale supporting the proposed changes to the Product Information. In particular, if the PIP is completed and the results of all studies are available, the applicant should discuss whether the generated data support or not the intended paediatric indication(s) stated in the PIP.
Inclusion of the results of all studies performed in compliance with an agreed Paediatric Investigation Plan requirement in the Product Information is a prerequisite for benefiting from the paediatric reward (Article 36(1) of Regulation (EC) No 1901/2006).
As for all applications including results of studies performed in compliance with an agreed PIP, the applicant should also include in Module 1.10 an overview table of the PIP results, indicating in which application(s) they were/are going to be submitted, status of the application(s), as well as their location in the present application.
In addition, in accordance with Article 8, the PIP or Waiver application and the related decision should cover both the new and existing indications, routes of administration and pharmaceutical forms of the authorised medicinal product, taking into account the Global Marketing Authorisation (GMA) concept together with the notion of ‘same marketing authorisation holder’. Further information can be found in the ‘Procedural advice on paediatric applications’, which is available on the Agency’s website under ‘Paediatric medicines’.
Those required data/documents should be included in Module 1.10 of the EU-CTD dossier.
The following types of application are exempted from the application of Article 8:
Furthermore, when planning submission of their marketing authorisation application, the applicant has to take into account also the need for a “PIP” compliance check to be done.
Such compliance check consists of verifying that the fulfilments of the measures as mentioned in the PIP decision including the timelines for the conduct of the studies or collection of the data are fulfilled. The compliance check procedure is explained in the document "Questions and answers on the procedure of paediatric-investigation-plan compliance verification at the European Medicines Agency, and paediatric rewards". Applicants are strongly recommended to apply for the compliance check before submission of the application to not delay the validation phase.
Further details on the format, timing and content of PIP or waiver applications as well as on the compliance check can be found in the Commission guideline. In addition, deadlines for submission of PIP or Waiver applications, application templates as well as Procedural Advice documents respectively regarding applications for PIPs, Waivers and Modifications and validation of new MAA, Variation/Extension applications and compliance check with an agreed PIP are available on the Agency's website in section “Paediatric medicines”.
References
The statement of compliance foreseen in Article 28(3) of Regulation (EC) No 1901/2006 is one of the prerequisites in order to be eligible for the paediatric rewards.
The following requirements have to be met for the paediatric investigation plan (PIP) compliance statement to be included in the technical dossier:
The MAH should submit the results of PIP studies or the remaining results if some were already submitted, as well as the elements mentioned above as part of a suitable variation or group of variations.
In addition, the MAH should clearly indicate in the cover letter that a PIP compliance statement is being claimed with the submission of this type II variation.
If all the above criteria are met, a PIP compliance statement will be included in the technical dossier.
The most appropriate variation classification will have to be determined based on the assessment required. A type II variation under one of the categories C.I.4 or C.I.6.a may be appropriate, depending on the proposed amendments to the product information. In some instances a type IB variation might be appropriate i.e. in situations when all data have already been assessed by the CHMP as part of a previous procedure and all results are already reflected in the product information.
For further details on the paediatric rewards please refer to “Questions and answers on the procedure of paediatric-investigation-plan compliance verification at the European Medicines Agency, and paediatric rewards”.
If the MAH wishes to withdraw their application for a type II variation during assessment, it should inform the Procedure Lead by providing a withdrawal letter stating that the MAH withdraws their application and indicating reasons for the withdrawal.
MAHs can address the withdrawal request to the CHMP Chairman at any point during the assessment (from validation of the application up until adoption of the CHMP opinion).
The withdrawal letter (as per the withdrawal letter template found in section 7 of the “Procedural advice on publication of information on withdrawals of applications” should be dated and signed by the MAH/authorised representative of the MAH and send to the EMA Procedure Lead, the Procedure Assistant and product shared mailbox.
Of note, the Agency will charge the fee for the validated variation, irrespective of its outcome (i.e., positive, negative or withdrawal) and publish information on withdrawn applications accordingly.
MAHs are informed that letters for withdrawal of extension of indication or modification of indication will be published on the EMA’s website (after redaction of protected personal data).
In addition, the MAH should submit within 15 days a consolidation sequence to remove the scientific and regulatory content of the withdrawn type II application from the eCTD structure and include the withdrawal letter in this sequence. The submission type should be “consolidating”.
However, not all of the content submitted in the withdrawn submission should be removed from the eCTD structure. It is useful to retain certain administrative information in the eCTD structure and some scientific or regulatory information may be used in future submissions. Therefore, the following rules should be applied:
Particular care should be taken to remove the versions of any labelling documents associated with the withdrawn variation.
References
The Product Lead (PL) is the primary contact for the applicant prior to submission and throughout the procedure for Type II variations related to the safety or efficacy of the medicinal product. However, if you have a procedural or regulatory pre-submission question when preparing your Type II variation application (Non-clinical/Clinical/RMP), please send it to us via email to IIquery@ema.europa.eu. Further, a dedicated pool of Quality Specialists will be dealing with Quality Type II variations and related queries. If you have a pre-submission question when preparing your Quality Type II variation application, please send it via email to the Quality Specialist assigned to your product.
The PL will serve as the main liaison person between the EMA product team, the Rapporteurs and the applicant. The PL, in close co-operation with the EMA product team and the rapporteurs, will ensure that the applicant is kept informed of all aspects related to the MAA evaluation of the application.
The applicant should contact the PL for all questions regarding the evaluation procedure, including
• Requests for scientific guidance in the pre-submission phase, such as the pre-submission meeting;
• Any type of procedural questions during the evaluation, such as availability of assessment reports and opinion documents;
• Discussion on timetables including requests for extension of clock-stops (Template for request of clock-stop extension) etc.
• Any question where guidance related to the evaluation procedure is needed. The PL will liaise with other EMA Product team members and redirect as appropriate.
At certain milestones during the evaluation procedure, the PL will contact the applicant for a direct exchange to facilitate the discussion on the scientific evaluation. These include:
• Preparation and conduct of clarification meetings (where applicant requests such meeting);
• Immediate feedback regarding scientific aspects from committee plenary discussions, where required;
• Expectations relating to the oral explanation, including topics to be addressed;
• Discussion of required post-authorisation measures;
• Late-stage revisions of the product information before adoption of the final opinion.
These interactions occur in close co-operation with the Rapporteurs. Occasionally other members from the EMA Product team may contact the applicant directly to facilitate the discussion on specific aspects.
When the applicant corresponds with other members of the EMA Product Team the PL should always be copied in the correspondence.
Please see other relevant questions and answers in the EMA pre-authorisation guidance “What is the role of the EMA product team?”, “Whom should I contact if I have a pre-submission question when preparing my Type II variation application (Non-clinical/Clinical/RMP)? and “Who is my contact at the European Medicines Agency during a marketing authorisation application (MAA) evaluation procedure?” and more information on ‘Contacting EMA: post-authorisation’.
If you cannot find the answer to your question in the Q&A when preparing your application:
The Agency aims to respond to your query within 10 working days. To help us deal with your enquiry, please provide as much information as possible including the name of the product in your correspondence. If you seek advice e.g. on the classification of change(s), or the acceptability of a single variation application vs a grouped variation application, please include your proposal. Your query will be channelled internally to the relevant service(s) that will respond to you.
Validation team: The validation of type II variations (Non-clinical/Clinical/RMP) will be handled by a dedicated team of Procedure Managers (PM). A PM will be nominated upon receipt of the variation application. You will be able to contact this PM directly if needed.