Type-IB variations: questions and answers
This page lists questions that marketing-authorisation holders (MAHs) may have on type-IB variations.
HumanRegulatory and procedural guidance
Updated on 26 November 2025:
- to update PDF versions of questions and answers and questions marked with "Rev. Nov 2025"
This page provides an overview of the European Medicines Agency's position on issues that are typically addressed in discussions or meetings with MAHs in the post-authorisation phase. Revised topics are marked 'New' or 'Rev.' upon publication.
These questions and answers have been produced for guidance only and should be read in conjunction with the rules governing medicinal products in the European Union, volume 2, notice to applicants.
MAHs must in all cases comply with the requirements of Community legislation. Provisions that extend to Iceland, Liechtenstein and Norway by virtue of the European Economic Area agreement are outlined in the relevant sections of the text.
Commission Regulation (EC) No 1234/2008 (‘the Variations Regulation’) defines a minor variation of Type IB as a variation which is neither a Type IA variation nor a Type II variation nor an Extension. Such minor variations must be notified to the relevant authority(ies) (competent authority of each Member State that granted a marketing authorisation /European Medicines Agency (‘the Agency’)) by the Marketing Authorisation Holder (MAH) before implementation, but do not require a formal approval. Upon acknowledgement of receipt of a valid notification and the notification of the start of the procedure, the MAH must wait a period of 30 days to ensure that the notification is deemed acceptable by the relevant authority(ies) before implementing the change (“Tell, Wait and Do” procedure).
The Commission guidelines on the details of the various categories of variations, on the operation of the procedures laid down in Chapters II, IIa, III and IV of Commission Regulation (EC) No 1234/2008 and on the documentation to be submitted pursuant to those procedures (‘the Variations Guidelines), contains examples of changes which are considered as Type IB variations. In addition, any change which is not an Extension and whose classification is not determined taking into account the Commission Guideline and the recommendations delivered pursuant to Article 5 of the Variations Regulation is considered a Type IB variation by default.
When one or more of the conditions established in the Classification Guideline for a Type IA variation are not met, the concerned change may be submitted as a Type IB variation unless the change is specifically classified as a major variation of Type II.
For changes which are submitted as default Type IB variations, the Agency will determine during validation whether the proposed classification as Type IB variation is appropriate before the start of the evaluation procedure (see also “How shall my Type IB variation be handled?”)
References
Upon validation of the notification by the Agency, the Rapporteur will be involved in the evaluation of Type IB variations “How shall my Type IB variation be handled (timetable)”?
The Co-Rapporteur is not involved in the assessment of Type IB variations.
MAHs may choose to group the submission of several Type IB variations for the same product into one notification. It is also possible for a MAH to group a Type IB variation with other variation(s) for the same product (e.g.
Allowed groupings are listed in Annex III of the Variations Regulation. Other groupings have to be agreed in advance with the Agency. Any proposal to group clinical and quality variations should be adequately justified.
Such grouped submissions will follow the review procedure of the highest variation in the group. Please also refer to “What type of variations can be grouped?”.
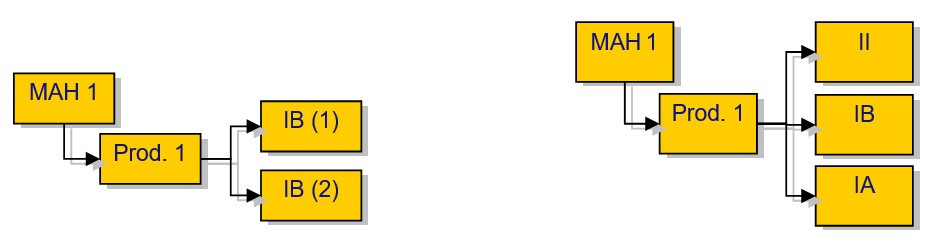
Where the same minor Type IB variation(s) affect more than one marketing authorisations from the same holder, the MAH shall submit these variations as one application for ‘worksharing’. Please also refer to “What is worksharing and what type of variations can be subject to worksharing?”.
References
Note: For information on applying the current and revised variations framework to Type IB variations please refer to the EMA’s dedicated webpage.[TG1] [AB2]
A Type IB variation notification should contain the elements listed in Annex IV of the Variations Regulation and should be presented in accordance with the appropriate headings and numbering of the EU-CTD format.
In order to help MAHs ensuring that their Type IB variations are complete and correct before submitting them to the Agency, it is strongly recommended to use the pre-notification checklist before submission of any Type IB variation.
In order to facilitate the completion of a correct application form before submission to the Agency, MAHs are advised to consult the EMA/CMDh Explanatory Notes on the Variation Application Form and the EMA Practical Guidance on the Application Form for Centralised Type IA and IB variations.
The Commission ‘Variations Guidelines’ further specifies which elements should be included in a Type IB variation notification:
Note: For additional guidance on the elements that should be included in a Type IB variation notification to be submitted from 15 January 2026 please refer to section 2 and the Annex of the EC Variations Guidelines (2025).
Grouped variations
For grouped variations concerning one marketing authorisation, all variations must be declared in the variation application form. The documentation requirements for each type of variation in the group must be adhered to. However, the supportive documentation for all variations concerned should be submitted as one integrated package (i.e. there is no need to submit a separate documentation package for each variation). The present-proposed section of the application form should clearly identify the relevant eCTD sections in support of each variation. For grouped variations please refer to “Can I group the submission of Type IB variations? Can they be grouped with other types of variations?”. For grouped variations concerning more than one marketing authorisation please refer to ”What is worksharing and what types of variations can be subject to worksharing?".
It should be noted that the responsibility for the quality of the submitted documentation lies with the MAH and is crucial to the overall process. The MAH is responsible for ensuring that the Type IB variation complies fully with the data and documentation requirements as specified in the Variations Guidelines. The MAH should pay particular attention to grouping of variations, for which each change should be clearly identified as well as the related supportive documentation. A confusing dossier presentation may delay the procedure.
For queries on technical matters please contact the EMA Service Desk. For procedural matters related to a Type IB notification for a specific product and in order to avoid rejection, please See Question 12. “Who should I contact if I have a question when preparing my application?”).
Variations to implement changes for generic/hybrid/biosimilar products
The Product Information (PI) for generic/hybrid/biosimilar medicinal products requires update following changes to the reference product via a post-authorisation regulatory procedure (e.g. safety update).
The EMA will no longer actively contact the MAHs of generic/hybrid/biosimilar medicinal products.
Instead, for centrally authorised medicinal products, the EMA will publish the reference medicinal product track changes PI in all EU languages on the reference medicinal product EPAR page. MAHs of generic/hybrid/biosimilar medicinal products of centrally authorised medicinal products are requested to monitor to EPAR updates of the reference medicinal product, download the track changes PI in all languages from the EPAR page following its publication and submit the appropriate variation(s) as soon as possible but no later than 2 months from the implementation of the changes in the PI as adopted for the reference product.
Should the relevant published track changes PI be superseded by a more recent EPAR update, the MAH of the generic/hybrid/biosimilar medicinal product should contact the Product Lead (PL) or Product Assistant (PA) of their product to request a copy of the track changes PI version which has not been downloaded on time. There is no need to file an Access to Documents (AtD) request.
For centrally authorised generic/hybrid/biosimilar medicinal products of nationally authorised reference medicinal products, the MAHs of generic/hybrid/biosimilar medicinal products are requested to follow the updates of the nationally authorised products PI and submit the relevant variation(s) to update the PI of the generic/hybrid/biosimilar medicinal product accordingly.
A submission of a type IB variation to implement changes for a generic/hybrid/biosimilar medicinal product, should include a detailed precise scope for the change(s) implemented, including relevant PI sections affected.
In addition, for generic/hybrid/biosimilar medicinal product of a centrally authorised reference medicinal product, a copy of the relevant English track changes PI(s) of the centrally authorised medicinal product should be included in the application, as well as a confirmation that the translations have been implemented in line with the translations of the reference medicinal product.
Submission of Type IB Notifications
Information is available on ‘Submitting a post-authorisation application’.
References
The linguistic review for IB variations will take place in parallel to the 30 day scientific assessment.
A linguistic review will, in general, be required for type IB variations with changes affecting the product information where the changes in wording have not previously undergone linguistic review.
Some examples of Type IB variations where a linguistic review will be performed include safety and efficacy Type IB variations affecting the product information, where the wording has not been provided by the Agency in all languages prior to the start of the procedure.
Some examples of Type IB variations where, in principle, a linguistic review will not be performed are:
References
Upon receipt of a Type IB notification, the Agency will handle the notification as follows:
a) Handling of Type IB variations included (‘foreseen’) in the Classification Guideline or covered by an Article 5 Recommendation:
Submission and validation
The Agency will check within 7 calendar days whether the variation is correct and complete (‘validation’) before the start of the evaluation procedure.
| Day | Action |
|---|---|
| Day x | Receipt of type IB variation |
| Day x+1 | Start of Agency validation |
| Day x+7 | Agency validation |
Issues identified during validation will be notified to the MAH via email. The MAH will be requested to provide responses to the issues raised within 5 working days. Delayed or insufficient responses will lead to complete or partial invalidation (in case of groupings) of the application as only one request for supplementary information will be issued during the validation phase.
The Agency will send to the MAH a confirmation of the positive outcome of the validation and the start date of the procedure.
Evaluation
| Day | Action |
|---|---|
| Day 1 | Start of evaluation |
| By day 20 | Internal circulation of assessment report* |
| By day 30 | (Non-)acceptance of the variation |
*Assessment Report will be sent to the applicant only at the end of the procedure not at Day 20 together with the IB notification.
Within 30 calendar days following the acknowledgement of receipt of a valid notification and the notification of the start of the procedure, the Agency will notify the MAH of the outcome of the procedure. The message will contain “Notification of a Type IB variation to the terms of the Marketing Authorisation” and the Assessment Report. If the Agency has not sent the holder its opinion on the notification within 30 calendar days, the notification shall be deemed acceptable.
Submission of amended notification (responses to Request for Supplementary Information (RSI)):
| Day | Action |
|---|---|
| By day 30 | Non-acceptance of the variation (RSI) |
| By day 60 | Submission of an amended notification (submission of responses to RSI by MAH) |
In case of an unfavourable outcome the MAH may, within 30 calendar days, amend the notification to take due account of the grounds for the non-acceptance of the variation. Clock-stops are not foreseen in a Type IB procedure. If the MAH does not amend the notification as requested, the notification shall be rejected.
Evaluation (assessment of responses to RSI)
| Day | Action |
|---|---|
| Day 60 | Receipt of an amended notification |
| By day 80 | Internal circulation of assessment report |
| By day 90 | Final (non-)acceptance of the variation |
Within 30 calendar days of receipt of the amended notification, the Agency will inform the MAH of its final (non-)acceptance of the variation and whether the Commission Decision granting the Marketing Authorisation requires any amendments.
Where the outcome of the procedure is favourable and the Commission Decision granting the Marketing Authorisation requires amendments, the Agency will inform the Commission accordingly.
Where Type IB Variations affect the Annexes to the Marketing Authorisation, such changes can be implemented without awaiting the update of the Commission Decision and the agreed change(s) should be included in the Annexes of any subsequent Regulatory Procedure.
b) Handling of Type IB variations claimed by the MAH to be IB variations by default:
The Agency will check within 7 calendar days whether the proposed change can be considered a minor variation of Type IB, and whether the notification is correct and complete (‘validation’) before the start of the evaluation procedure. In exceptional cases, the Agency may have to consult with the Rapporteur on the appropriate classification of the variation, which may lead to a slightly longer validation period (up to 10 working days).
When the Agency is of the opinion that the proposed variation may have a significant impact on the quality, safety or efficacy of the medicinal product, the MAH will be notified that the applied change cannot be handled as a Type IB and that the variation will have to be reclassified as a Type II variation. As a consequence, the MAH will be requested to revise and supplement its variation application so that the requirements for a Type II variation application are met.
Following receipt of the valid revised variation application, a Type II assessment procedure will be initiated according to the Agency procedural timetables for Type II variation.
When the Agency is of the opinion that the proposed variation can be considered a Type IB variation, the MAH will be informed of the outcome of the validation and of the start date of the procedure. The Type IB notification will be handled as set-out in section a) above.
c) Handling of Groupings of Minor Variations (Type IB/Type IA)
For grouping of minor variations, where not all of the changes applied for can be positively validated, all valid and not valid variations will be clearly listed in the validation outcome correspondence.
Where a Type IB by default variation, within a group of variations, has to be reclassified as a Type II variation, the MAH will be requested to confirm whether this variation should remain in the group. If confirmed, the whole group will be handled as a Type II variation, as set out in b) above.
Where several Type IB variations are submitted as part of one notification, it will be clearly specified in the final Agency notification which variation(s) have been accepted or rejected following assessment, unless some of the variations have been withdrawn by the MAH during the procedure (see grouping Q&A).
There is no fee payable for Type IB variations.
References
For information concerning submission of mock-ups and specimens in the framework of post-authorisation procedures, section 3.4 Other post-authorisation procedures in the document Checking process of mock-ups and specimens of outer / immediate labelling and package leaflets of human medicinal products in the centralised procedure'.
In case the Type IB notification affects any of the Annexes, i.e. Annex A, SmPC, Annex II, labelling and/or package leaflet, the affected revised product information Annexes must be submitted as follows:
At submission, the MAH should provide:
If Annex A is affected, please submit all EEA language versions in word (highlighted) electronically and in PDF (clean) in eCTD.
At submission, the MAH should provide:
If Annex A is affected, please submit all EEA language versions in word (highlighted) electronically and in PDF (clean) in eCTD.
Please be reminded that in accordance with Union data protection requirements, no personal data should be included in the annotated PIs. This applies to the English version as well as all the other translation versions. The annotated product information files must include the statement containing the procedure number(s) and may be published on the EMA website as part of the product EPAR page. Please submit annotated PIs in an anonymised format (i.e. names of the reviewers removed from the track-changes). If you do not wish to do so, please ensure that the individuals whose data is included consented to its sharing with EMA, the publication on the EMA website and its further sharing by EMA with third parties such as other marketing authorisation applicants, marketing authorisation holders and National Competent Authorities, as relevant. EMA expressly disclaims any liability or accountability for the presence of unnecessary personal data in the annotated PI submitted by the marketing authorisation holder.
Upon validation of the procedure the MAH will receive the timetable for the submission of the translations of the product information for linguistic review.
In all cases the 'complete set of Annexes' includes Annex A (if applicable), I, II, IIIA and IIIB i.e. all authorised presentations (if applicable), SmPC, labelling and PL texts for all strengths and pharmaceutical forms of the product concerned, as well as Annex II. The complete set of Annexes must be presented sequentially (i.e. Annex I, II, IIIA, IIIB) as one document for each official EU language. Page numbering should start with "1" (bottom, centre) on the title page of Annex I. The 'QRD Convention' published on the Agency website should be followed. When submitting the full set of Annexes in PDF format, this should be accompanied by the completed Checklist for the submission of product information annexes and Annex A (if applicable) for minor procedures without linguistic review - human which provides User guide on how to generate PDF versions of the product information - human on how to correctly prepare the PDF versions.
The electronic copy of all languages should be provided as part of the variation application. Highlighted changes should be indicated via 'Tools – Track Changes'. Clean versions should have all changes 'accepted'.
Icelandic and Norwegian language versions must always be included.
The Annexes provided should only reflect the changes introduced by the Variation(s) concerned. However, in exceptional cases where MAHs take the opportunity to introduce minor linguistic amendments in the texts this should be clearly mentioned in the cover letter and in the scope section of the application form (see also “What can be considered an editorial change and how can it be submitted as part of a type IA/IB/II variation?”).
In addition, the section “present/proposed” in the application form should clearly list the minor linguistic amendments introduced for each language. Alternatively, such listing may be provided as a separate document attached to the application form. Any changes not listed, will not be considered as part of the variation application.
In such cases and in cases where any other ongoing procedure(s) may affect the product information Annexes, the MAH is advised to contact the Agency in advance of submission or finalisation of the procedure(s) concerned.
For Type IB variations affecting Annex A where the variation introduces a new EU sub-number, the sub-number should be included in the Annex A and in the product information texts as part of the variation application (see also “How to obtain new EU sub-numbers for a Type IB variation concerning an additional presentation? (e.g. new pack-size)?”).
Similarly, in case of a deletion of a pharmaceutical form/strength(s), the amended Annex A and product information Annexes should be provided as part of the Variation application.
Any changes in the number of units of medicinal product or medical device being an integral part of the medicinal product (e.g. prefilled syringes) will trigger a different EU number.
Differentiation should be made between the addition of a presentation where the two presentations will co-exist on the market on a long-term basis versus a replacement of a presentation where the new presentation will replace the previous one (it is expected that for a limited period of time, the two presentations may co-exist on the market until the stock of the previous presentation runs out).
In principle, a replacement of one presentation by another presentation does not trigger a new EU number, unless the number of units of medicinal product or medical device being an integral part of the medicinal product (e.g. prefilled syringes) is changed.
Examples of changes in presentations for replacement, not triggering a new EU number (this is not an exhaustive list):
Examples of changes in presentations for replacement, triggering a new EU number (this is not an exhaustive list):
In case of addition, as the presentations will co-exist on the market, two packs with different contents cannot be covered by the same EU number and will be considered as different presentations.
Changes in the number of any unit (not restricted to the medicinal product) or changes in the specifications of any unit (not restricted to the medicinal product) contained in the pack will trigger a new EU number.
Examples of changes that will trigger new EU numbers (this is not an exhaustive list):
In the specific case of a Type IB Variation for an additional presentation, the new EU marketing authorisation sub-number should be requested from the Agency before submission.
The request should be sent together with a Checklist for requesting new EU sub-numbers (type IAIN and Type IB lead procedures only) and a draft Annex A (in English only) through the EMA Service Desk, selecting the tab “Business Services”, category “Human Regulatory”. The subcategory to be selected is “Post-authorisation - Human”, followed by the sub-option “New EU number request”. The request should be made at least 5 working days in advance of the intended submission of the variation. Once a number has been allocated, this number should subsequently be included in the Annex A and Product Information Annexes submitted together with the Variation notification.
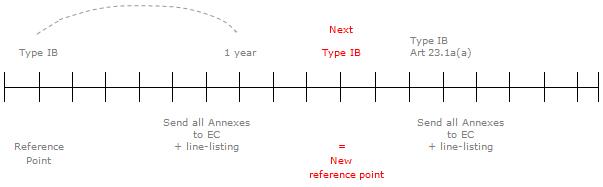
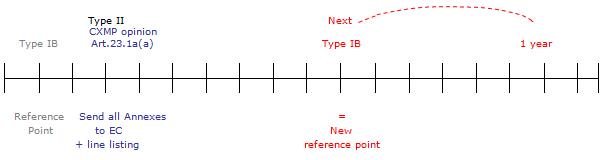
For type-IB variations affecting the annexes to the Commission decision, the Commission decision will generally be updated within one year, unless the type-IB variation concerns any of the changes listed in Article 23.1a(a), for which the Commission decision will be updated within two months. This would include variations related to the addition of a new therapeutic indication or modification of an existing one, addition of a new contraindication or change in posology. It is expected that such variations would be processed as type-IB variations mainly in the framework of generics and hybrids following changes to the product information of the reference medicinal product.
However, all type-IB variations affecting the annexes can be implemented without awaiting the update of the marketing authorisation and the agreed type-IB changes should be included in the annexes of any subsequent regulatory procedure.
For type-IB variations subject to yearly update of the respective Commission decision, at the end of this yearly period, the Agency will send the complete set of annexes, based on the latest approved annexes and reflecting the type-IB changes introduced during the past year as well as a line-listing of those variations pending update of the Commission decision.
Where a notification contained several type-IB variations concerning one marketing authorisation, the Commission will update the marketing authorisation with one single decision to cover all the approved minor variations.
However, where a notification or opinion affecting the annexes that is followed by an immediate Commission decision is transmitted to the Commission within this yearly period, the changes of the type-IB notifications concerned will already be included in the annexes to the notification or opinion and will consequently be reflected in the resulting Commission decision. This Commission decision will therefore replace the yearly updating of the marketing authorisation for the type-IB notifications concerned.
On the occasion of the next type-IB variation affecting the annexes, the procedure outlined above will be repeated based on the new reference point of the next type IB concerned.
See the diagram below:
If you cannot find the answer to your question in the Q&A when preparing your application, please raise a ticket via the EMA Service Desk, selecting the tab “Business Services”, category “Human Regulatory”. The subcategory to be selected is “Post-authorisation - Human”, followed by the sub-option: “Variation IB A&B scopes queries” or “Variation IB C scopes queries”.
The Agency aims to respond to your query within 10 working days. To help us deal with your enquiry, please provide as much information as possible including the name of the product in your correspondence.
You should submit your query once (please avoid opening multiple tickets with the same question, tickets can always be reopened in case of follow-up) and it is important that you submit it using the applicable type of question and sub-option. If you are uncertain on a classification of a variation as Type IB or Type IA, please use only one of the sub-option “Variation IA queries” or “Variation IB A&B scopes queries” or “Variation IB C scopes queries”. If you seek advice on the classification of change(s), please include your proposal for classification. Your query will be channelled internally to the relevant service(s) that will respond to you.
If you do not have an EMA Account, you may create one via the EMA Account Management portal. For further information or guidance about how to create an EMA Account reference the guidance "Create an EMA Account".
Type IB variations will be handled by a dedicated team of Procedure Managers (PM). A PM will be nominated upon receipt of the variation. You will be able to contact this PM throughout the procedure via the IRIS case. If you have any comments or questions once the procedure has started, please send them to the IRIS case rather than through ServiceDesk, so that they can be replied directly by the PM allocated to the procedure.