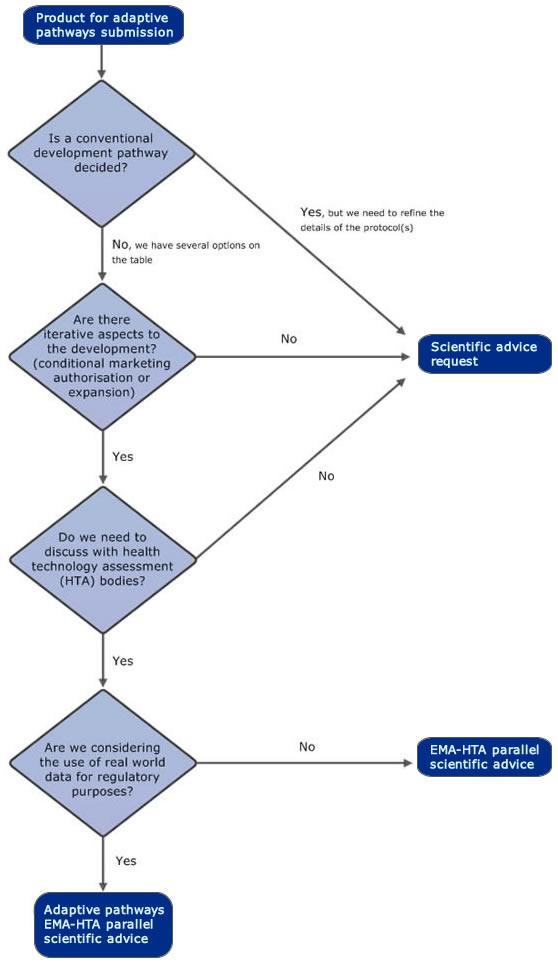
Adaptive pathways
The adaptive pathways approach is part of the European Medicines Agency's (EMA) efforts to improve timely access for patients to new medicines. Adaptive pathways is a scientific concept for medicine development and data generation which allows for early and progressive patient access to a medicine. The approach makes use of the existing European Union (EU) regulatory framework for medicines.
HumanEarly accessRegulatory and procedural guidanceResearch and development