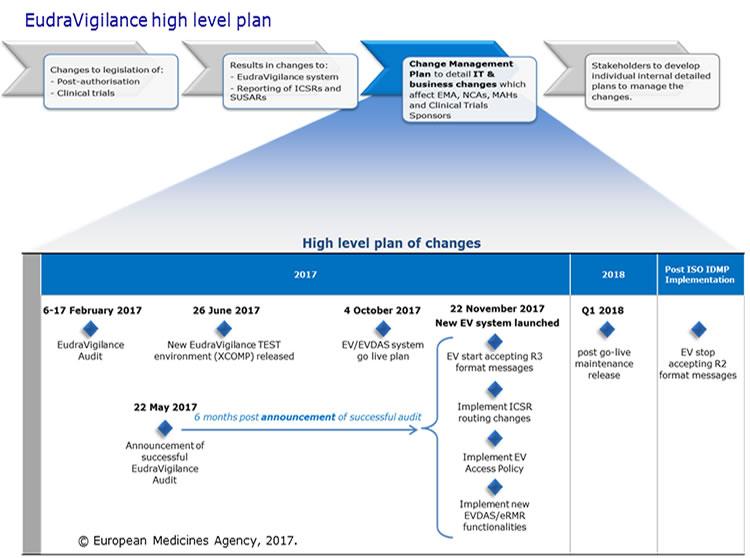
Change management for the EudraVigilance system
The European Medicines Agency (EMA) launched an enhanced EudraVigilance system in November 2017, to support the changes to electronic reporting requirements for suspected adverse reactions brought about by the European Union (EU) pharmacovigilance legislation.
HumanRegulatory and procedural guidancePharmacovigilanceResearch and development