European SME Week - supporting a major driver of innovation
NewsCorporateSME
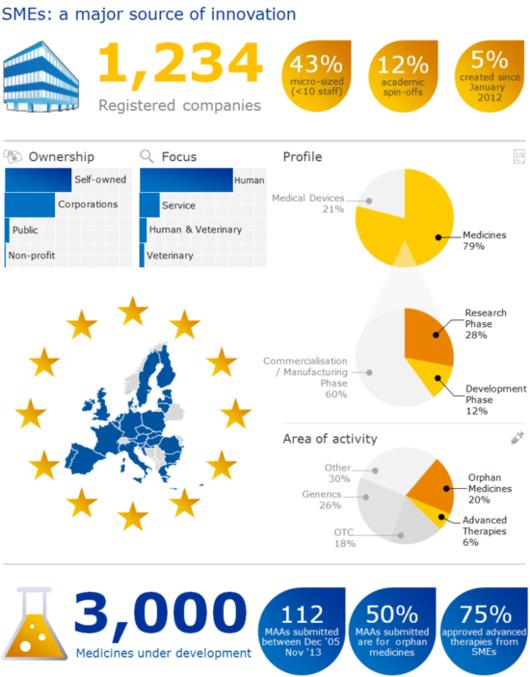
The European Medicines Agency (EMA) and the European Union (EU) recognise that micro, small and medium-sized enterprises (SMEs) are a motor of innovation in the EU. Recognising their role in the development of new medicines, the EMA has a programme in place to support SMEs throughout all stages of medicine development.
The Agency put the SME initiative in place in December 2005 to promote innovation and development of medicines by SMEs. The SME Office is a dedicated structure that provides active regulatory, financial and administrative support to registered SMEs in the development of their medicines.
More than 1,200 SMEs are now registered with the Agency. Together, the registered SMEs have more than 3,000 medicines at development stage. SME applicants account for only a small proportion (around 11%) of centralised marketing-authorisation applications for human and veterinary medicines. However, this figure does not include medicines that originate from SMEs but belong to larger pharmaceutical companies at the stage of licensing due to mergers/acquisitions and out-licensing activities.
SMEs are a major source of medicines for the treatment of rare diseases and therefore greatly contribute to improve public health. Among the 14 applications submitted by SMEs currently under evaluation by the CHMP, eight are for orphan medicines.
SMEs are also the main commercial developers of advanced therapy medicinal products (ATMPs), innovative medicines that are derived from gene therapy, cell therapy or tissue engineering and which offer groundbreaking new opportunities for the treatment of disease and injury. Of note, three out of the four advanced therapies that have been approved in the EU so far have been developed by SMEs. A further ATMP submitted by an SME is currently under evaluation by the CHMP.
Financial support for SMEs
Access to capital remains a key concern for SMEs. A number of initiatives to support financing of SMEs are available at the EU-level as well as research funding opportunities from the European Commission. An Overview of current initiatives at European Union level to assist micro, small and medium-sized enterprises with financing is available on the Agency's website.
In this area, a new European instrument, Horizon 2020, which will replace the Seventh Framework Programme for Research and Technological Development (FP7), was approved at the European Parliament on 21 November 2013. Horizon 2020 will include a dedicated instrument for SMEs. As part of this programme, for the first time SMEs will be able to apply for funding for their innovative research and development projects as single companies and not only as part of a consortium.
SMEs are advised to contact the Enterprise Europe Network to check whether any EU or national programmes are available to help with the financing of their specific project/company. The Network can guide a company through the range of EU and local programmes which may be available in a specific country.
On Tuesday 26 November 2013, as part of the European SME Week, the Agency will provide an overview of the tools that the Agency has made available to SMEs, and the industry in general, to support them in the development of their medicines and increase their chances of success.
More on European SME Week
European SME Week promotes enterprise across Europe by supporting the work of SMEs. It is co-ordinated by the European Commission. Its aims include: