Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 13-16 September 2021
NewsHumanCOVID-19MedicinesReferrals
EMA’s human medicines committee (CHMP) recommended nine medicines for approval at its September 2021 meeting.
The Committee adopted a positive opinion for Artesunate Amivas* (artesunate), for the initial treatment of severe malaria in adults and children. This medicine has an orphan designation because malaria has a low prevalence in the European Union.
The CHMP recommended granting a marketing authorisation for Brukinsa* (zanubrutinib), for the treatment of Waldenström’s macroglobulinaemia.
The Committee adopted a positive opinion, recommending the granting of a conditional marketing authorisation for Gavreto (pralsetinib) for the treatment of non-small cell lung cancer.
The CHMP recommended granting a marketing authorisation for Qinlock* (ripretinib) for the treatment of advanced gastrointestinal stromal tumour (GIST).
Vumerity (diroximel fumarate) was granted a positive opinion for the treatment of adult patients with relapsing remitting multiple sclerosis.
Two biosimilar medicines were recommended for approval by the Committee: Hukyndra and Libmyris (both containing adalimumab) for the treatment of inflammatory auto-immune disorders such as arthritis psoriasis, inflammatory bowel disease or uveitis. A biosimilar medicine is a biological medicine that is highly similar to another biological medicine that is already authorised for use.
Two generic medicines received a positive opinion from the Committee: Sugammadex Mylan (sugammadex) intended for the reversal of induced neuromuscular blockade and Rivaroxaban Mylan (rivaroxaban), an anti-coagulant intended for the prevention and treatment of atherothrombotic and venous thromboembolic events in certain at-risk patients.
The CHMP recommended the refusal of a marketing authorisation for Raylumis (tanezumab). Raylumis was intended for the treatment of pain associated with osteoarthritis.
For more information on this negative opinion, see the question-and-answer document in the grid below.
The Committee recommended granting three extensions of indication for Nucala (mepolizumab), including the extension for the treatment of eosinophilic granulomatosis with polyangiitis. For more information on this extension, see the news announcement in the grid below.
Other extensions of indication recommended by the Committee were for Firmagon, Jyseleca, Keytruda, Noxafil, Opdivo, Segluromet, Steglatro and Zepatier.
The CHMP has concluded its further analysis of data on the risk of unusual blood clots linked to low levels of blood platelets (thrombosis with thrombocytopenia syndrome, TTS) and on the use of a second dose of Vaxzevria, reinforcing its interim opinion of April 2021. A scientific opinion on these points was requested by the European Commission following the initial reports of TTS associated with the vaccine. EMA analysed all the available data, including the latest TTS data from spontaneous reports in EudraVigilance, detailed vaccination data from Member States and an additional commissioned study of the risk of blood clots that was reviewed in detail by EMA’s safety committee (PRAC).
The evidence did not allow EMA to identify particular risk factors that make TTS more likely. Although spontaneous reports when put in relation to the exposure have suggested that the risk may be higher in women and in younger adults, and lower after the second compared to the first dose, the limitations of the way the data is collected mean that none of these differences could be confirmed.
EMA’s recommendation remains to continue giving a second dose of Vaxzevria between 4 and 12 weeks after the first, in line with the product information. There is no evidence that delaying the second dose has any influence on the risk of TTS. Where a second dose of Vaxzevria is not given, no definitive recommendations on the use of a different vaccine for the second dose can be made at present (see EMA/ECDC Joint Statement).
The CHMP’s detailed assessment report will be published shortly.
The applicant for Nouryant (istradefylline) has requested a re-examination of the Committee’s opinion for this medicine adopted at its July 2021 meeting. Upon receipt of the grounds of the request, the Agency will re-examine its opinion and issue a final recommendation.
The applicant for Nexviadyme avalglucosidase alfa) has requested a re-examination of the Committee’s opinion for this medicine adopted at its July 2021 meeting. Upon receipt of the grounds of the request, the Agency will re-examine its opinion and issue a final recommendation.
Applications for an initial marketing authorisation for four medicines were withdrawn: Livmarli (maralixibat) for the treatment of progressive familial intrahepatic cholestasis type 2 (PFIC2) in patients 1 year of age and older; Oportuzumab monatox DLRC Pharma Services (oportuzumab monatox) for the treatment and prevention of recurrence of cancer of the bladder and the prevention of recurrence of papillary tumours; Sildenafil FGK (sildenafil) for the treatment of erectile dysfunction in adult men; Teriparatide Cinnagen (teriparatide) for the treatment osteoporosis.
Question-and-answer documents on the withdrawals are available in the grid below.
The CHMP has concluded its analysis on the presence of impurity N-nitroso-varenicline in Champix at levels above those considered acceptable for EU medicines. Champix should conform to a limit of impurity calculated based on compound-specific ICH M7 principles for a lifetime exposure. The company will now be asked to vary its licence to comply with the set requirements. As a precautionary measure the company that markets Champix had already recalled several batches in June 2021 and paused distribution. The Direct healthcare professional communication (DHPC): Champix (varenicline) - lots to be recalled due to presence of impurity N-nitroso-varenicline above the Pfizer acceptable daily intake limit direct healthcare professional communication (DHPC) is being revised informing healthcare professionals that further shortages are anticipated. The updated DHPC will be published in the coming days on the following page. As part of the assessment, CHMP considered the criticality of Champix in the context of its current therapeutic use and the availability of alternative treatment options. The CHMP concluded that higher impurity limits were not acceptable as the product was not critical and its absence from the EU market would not create a concern in terms of public health. Patients should not stop taking Champix without first consulting their healthcare professional and talk to them if they have any questions or concerns.
The CHMP re-elected Harald Enzmann as its chair for a second three-year term, starting in September 2021.
The agenda of the September 2021 CHMP meeting is published on EMA's website. Minutes of the July 2021 CHMP meeting will be published in the coming weeks.
Key figures from the September 2021 CHMP meeting are represented in the graphic below.
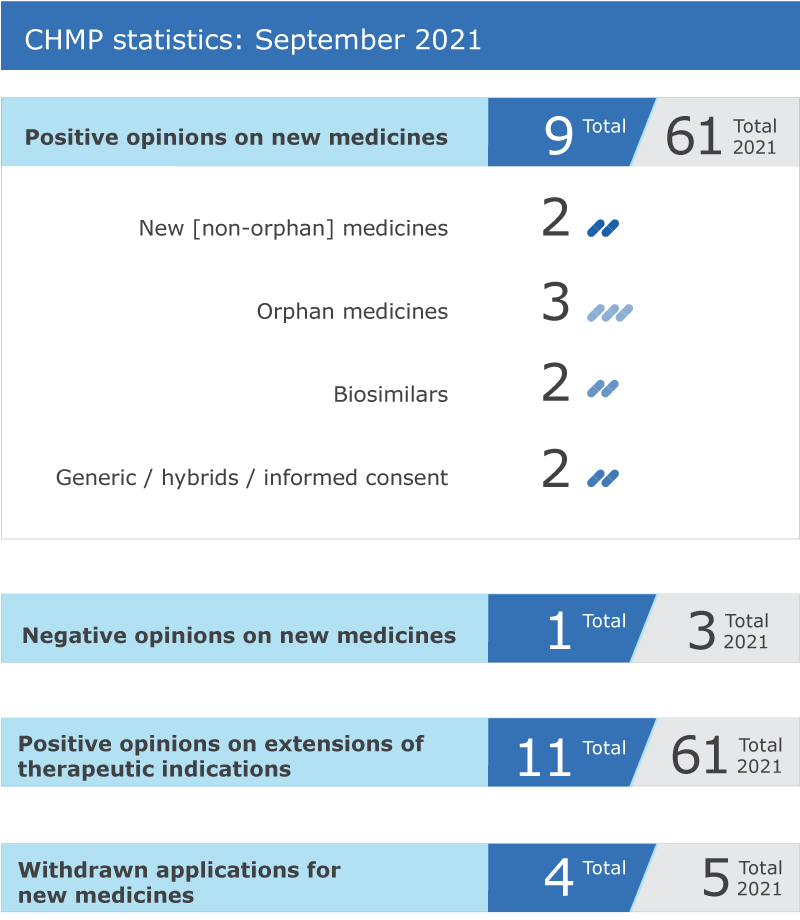
*This product was designated as an orphan medicine during its development. Orphan designations are reviewed by EMA's Committee for Orphan Medicinal Products (COMP) at the time of approval to determine whether the information available to date allows maintaining the medicine’s orphan status and granting the medicine ten years of market exclusivity.
| Name of medicine | Artesunate Amivas |
| International non-proprietary name (INN) | artesunate |
| Marketing-authorisation applicant | Amivas Ireland Ltd |
| Therapeutic indication | Initial treatment of severe malaria in adults and children |
| More information | Artesunate Amivas: Pending EC decision |
| Name of medicine | Brukinsa |
| INN | zanubrutinib |
| Marketing-authorisation applicant | BeiGene Ireland Ltd |
| Therapeutic indication | Treatment of Waldenström’s macroglobulinaemia (WM) |
| More information | Brukinsa: Pending EC decision |
| Name of medicine | Gavreto |
| INN | pralsetinib |
| Marketing-authorisation applicant | Roche Registration GmbH |
| Therapeutic indication | Treatment of non-small cell lung cancer (NSCLC) |
| More information | Gavreto: Pending EC decision |
| Name of medicine | Qinlock |
| INN | ripretinib |
| Marketing-authorisation applicant | Deciphera Pharmaceuticals (Netherlands) B.V. |
| Therapeutic indication | Treatment of advanced gastrointestinal stromal tumour (GIST) |
| More information | Qinlock: Pending EC decision |
| Name of medicine | Vumerity |
| INN | diroximel fumarate |
| Marketing-authorisation applicant | Biogen Netherlands B.V. |
| Therapeutic indication | Treatment of adult patients with relapsing remitting multiple sclerosis |
| More information | Vumerity: Pending EC decision |
| Name of medicine | Raylumis |
| INN | tanezumab |
| Marketing-authorisation applicant | Pfizer Europe MA EEIG |
| Therapeutic indication | Treatment of pain associated with osteoarthritis |
| More information | Raylumis: Pending EC decision |
| Name of medicine | Nouryant |
| INN | istradefylline |
| Marketing-authorisation applicant | Kyowa Kirin Holdings B.V. |
| Therapeutic indication | Adjunctive treatment to levodopa-based regimens in patients with Parkinson’s disease |
| More information | Nouryant: Pending EC decision |
| Name of medicine | Nexviadyme |
| INN | avalglucosidase alfa |
| Marketing-authorisation applicant | Genzyme Europe BV |
| Therapeutic indication | Long-term enzyme replacement therapy for the treatment of patients with Pompe disease |
| More information | Nexviadyme: Pending EC decision |
| Name of medicine | Rivaroxaban Mylan |
| INN | rivaroxaban |
| Marketing-authorisation applicant | Mylan Ireland Limited |
| Therapeutic indication | Prevention and treatment of atherothrombotic and venous thromboembolic events |
| More information | Rivaroxaban Mylan: Pending EC decision |
| Name of medicine | Sugammadex Mylan |
| INN | sugammadex |
| Marketing-authorisation applicant | Mylan Ireland Limited |
| Therapeutic indication | Reversal of induced neuromuscular blockade |
| More information | Sugammadex Mylan: Pending EC decision |
| Name of medicine | Hukyndra |
| INN | adalimumab |
| Marketing-authorisation applicant | STADA Arzneimittel AG |
| Therapeutic indication | Treatment of inflammatory auto-immune disorders |
| More information | Hukyndra: Pending EC decision |
| Name of medicine | Libmyris |
| INN | adalimumab |
| Marketing-authorisation applicant | STADA Arzneimittel AG |
| Therapeutic indication | Treatment of inflammatory auto-immune disorders |
| More information | Libmyris: Pending EC decision |
| Name of medicine | Firmagon |
| INN | degarelix |
| Marketing-authorisation holder | Ferring Pharmaceuticals A/S |
| More information | Firmagon: Pending EC decision |
| Name of medicine | Jyseleca |
| INN | filgotinib |
| Marketing-authorisation holder | Gilead Sciences Ireland UC |
| More information | Jyseleca: Pending EC decision |
| Name of medicine | Keytruda |
| INN | pembrolizumab |
| Marketing-authorisation holder | Merck Sharp & Dohme B.V. |
| More information | Keytruda: Pending EC decision |
| Name of medicine | Noxafil |
| INN | posaconazole |
| Marketing-authorisation holder | Merck Sharp & Dohme B.V. |
| More information | Noxafil: Pending EC decision |
| Name of medicine | Nucala |
| INN | mepolizumab |
| Marketing-authorisation holder | GlaxoSmithKline Trading Services |
| More information | Nucala: Pending EC decision
News announcement: New add-on treatment for rare autoimmune inflammatory disorder |
| Name of medicine | Opdivo |
| INN | nivolumab |
| Marketing-authorisation holder | Bristol-Myers Squibb Pharma EEIG |
| More information | Opdivo: Pending EC decision |
| Name of medicine | Segluromet |
| INN | ertugliflozin / metformin hydrochloride |
| Marketing-authorisation holder | Merck Sharp & Dohme B.V. |
| More information | Segluromet: Pending EC decision |
| Name of medicine | Steglatro |
| INN | ertugliflozin |
| Marketing-authorisation holder | Merck Sharp & Dohme B.V. |
| More information | Steglatro: Pending EC decision |
| Name of medicine | Zepatier |
| INN | elbasvir / grazoprevir |
| Marketing-authorisation holder | Merck Sharp & Dohme B.V. |
| More information | Zepatier: Pending EC decision |
| Name of medicine | Adempas |
| INN | riociguat |
| Marketing-authorisation applicant | Bayer AG |
| New contraindication | Concomitant use with other soluble guanylate cyclase stimulators |
| More information | Adempas: Pending EC decision |
| Name of medicine | Byannli (previously Paliperidone Janssen-Cilag International) |
| INN | paliperidone |
| Marketing-authorisation holder | Janssen-Cilag International N.V. |
| More information | Byannli: Pending EC decision |
| Name of medicine | Livmarli |
| INN | maralixibat |
| More information | Livmarli: Withdrawn application |
| Name of medicine | Oportuzumab monatox DLRC Pharma Services |
| INN | oportuzumab monatox |
| More information | Oportuzumab monatox DLRC Pharma Services: Withdrawn application |
| Name of medicine | Sildenafil FGK |
| INN | sildenafil |
| More information | Sildenafil FGK: Withdrawn application |
| Name of medicine | Teriparatide Cinnagen |
| INN | teriparatide |
| More information | Teriparatide Cinnagen: Withdrawn application |