Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 16-19 November 2015
NewsHumanCorporate
Ten medicines, including a first-in-class orphan medicine for narcolepsy, recommended for authorisation in the EU
The European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) recommended ten medicines for marketing authorisation at its November 2015 meeting.
The CHMP recommended granting a marketing authorisation for Wakix (pitolisant) for the treatment of narcolepsy. Narcolepsy is a rare, long-term sleep disorder which affects the brain's ability to regulate the normal sleep-wake cycle, and may occur with or without cataplexy (sudden severe muscle weakness or loss of muscle control). Wakix, a first-in-class medicine, has an orphan designation. For more information, please see the press release in the grid below.
The CHMP recommended granting a marketing authorisation for two blood cancer medicines: Oncaspar (pegaspargase) and Spectrila (asparaginase), both for the treatment of acute lymphoblastic leukaemia. Spectrila has an orphan designation.
Benepali (etanercept), a biosimilar, received a positive opinion for the treatment of rheumatoid arthritis, psoriatic arthritis, axial spondyloarthritis and plaque psoriasis.
Briviact (brivaracetam) was recommended for marketing authorisation by the Committee for the treatment of partial-onset epilepsy seizures.
The CHMP recommended granting a marketing authorisation to Episalvan for the treatment of partial thickness wounds in adults. The active substance of Episalvan is birch bark extract.
Three generic medicines received positive opinions from the Committee: Pemetrexed Accord (pemetrexed) for the treatment of unresectable malignant pleural mesothelioma and locally advanced or metastatic non-small cell lung cancer, Lopinavir / Ritonavir Mylan (lopinavir / ritonavir) for the treatment of human immunodeficiency virus (HIV) infection in adults, adolescents and children above the age of two years and Eptifibatide Accord (eptifibatide) for the prevention of early myocardial infarction.
One hybrid medicine, Pemetrexed Actavis (pemetrexed), received a positive opinion for the treatment of unresectable malignant pleural mesothelioma and locally advanced or metastatic non-small cell lung cancer. Hybrid applications rely in part on the results of studies carried out with a reference product and in part on new data.
Negative opinion on new medicine
The CHMP adopted a negative opinion for Solumarv (insulin human) which was expected to be used to treat patients with diabetes who require insulin to control their blood sugar levels. Solumarv was developed as a biosimilar medicine. For more information, please see the questions-and-answers document in the grid below.
Three recommendations on extensions of therapeutic indication
The Committee recommended extensions of indication for Cimzia and Zutectra.
The Committee also recommended extending the use of Pyramax, an artemisinin combination therapy for malaria which was first evaluated in 2012 under EMA's Article 58 programme. This allows the CHMP to assess and give a scientific opinion in cooperation with the World Health Organization (WHO) for medicines intended exclusively for markets outside the European Union (EU). Through this mechanism, regulators outside the EU can use the CHMP assessment as part of their national authorisation process.
Pyramax is currently available as a film-coated tablet to treat malaria caused by P. falciparum and P. vivax in adults and children over 20 kg. These species of the malaria parasite account for the majority of cases.
The CHMP recommends to add a new pharmaceutical form, granules for oral suspension, and to extend the use of Pyramax in this formulation to children and infants weighing 5 kg to less than 20 kg. This provides an age-appropriate formulation for very small children.
The CHMP also recommended an extension of the indication for Pyramax to remove restrictions on repeated courses of treatment in patients and on its use only in areas of low malaria transmission with evidence of artemisinin resistance.
Outcome of review of human papillomavirus (HPV) vaccines
The CHMP has completed the review of the evidence surrounding reports of two syndromes, complex regional pain syndrome (CRPS) and postural orthostatic tachycardia syndrome (POTS) in young women given human papillomavirus (HPV) vaccines. After due consideration, the CHMP confirmed the recent recommendation from EMA's Pharmacovigilance Risk Assessment Committee (PRAC) that the available evidence does not support a causal link between the vaccines and development of CRPS or POTS. Please refer to the public health communication in the grid below for more information.
Agenda and minutes
The agenda of the November 2015 meeting is published on EMA's website. Minutes of the October 2015 CHMP meeting will be published next week.
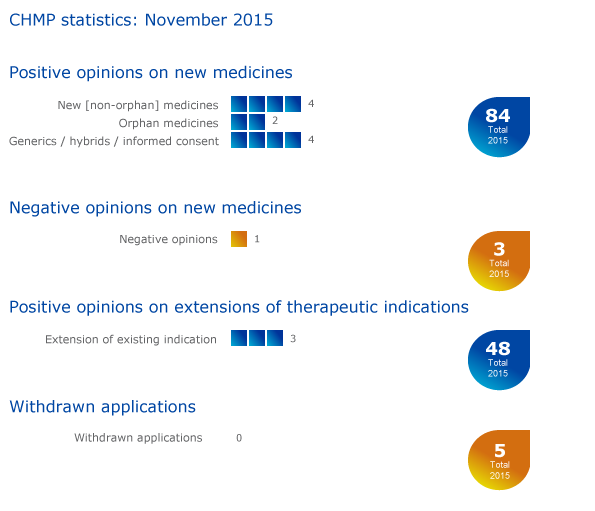
CHMP statistics
Key figures from the November 2015 CHMP meeting are represented in the graphic below.
More information on this, and all other outcomes of the CHMP's November 2015 meeting, is available in the grid below.
| Name of medicine | Benepali |
|---|---|
| International non-proprietary name (INN) | etanercept |
| Marketing-authorisation applicant | Samsung Bioepis UK Limited (SBUK) |
| Therapeutic indication | Treatment of rheumatoid arthritis, psoriatic arthritis, axial spondyloarthritis and plaque psoriasis |
| More information | CHMP summary of positive opinion for Benepali |
| Name of medicine | Briviact |
|---|---|
| International non-proprietary name (INN) | brivaracetam |
| Marketing-authorisation applicant | UCB Pharma SA |
| Therapeutic indication | Treatment of partial-onset seizures |
| More information | CHMP summary of positive opinion for Briviact |
| Name of medicine | Episalvan |
|---|---|
| International non-proprietary name (INN) | birch bark extract |
| Marketing-authorisation applicant | Birken AG |
| Therapeutic indication | Treatment of partial thickness wounds |
| More information | CHMP summary of opinion for Episalvan |
| Name of medicine | Oncaspar |
|---|---|
| International non-proprietary name (INN) | pegaspargase |
| Marketing-authorisation applicant | Baxalta Innovations GmbH |
| Therapeutic indication | Treatment of acute lymphoblastic leukaemia |
| More information | CHMP summary of opinion for Oncaspar |
| Name of medicine | Spectrila |
|---|---|
| International non-proprietary name (INN) | asparaginase |
| Marketing-authorisation applicant | medac Gesellschaft fuer klinische Spezialpraeparate mbH |
| Therapeutic indication | Treatment of acute lymphoblastic leukaemia |
| More information | CHMP summary of positive opinion for Spectrila |
| Name of medicine | Wakix |
|---|---|
| International non-proprietary name (INN) | pitolisant |
| Marketing-authorisation applicant | Bioprojet Pharma |
| Therapeutic indication | Treatment of narcolepsy |
| More information |
CHMP summary of opinion for Wakix
Press release: Narcolepsy treatment recommended for approval |
| Name of medicine | Eptifibatide Accord |
|---|---|
| International non-proprietary name (INN) | eptifibatide |
| Marketing-authorisation applicant | Accord Healthcare Limited |
| Therapeutic indication | Prevention of early myocardial infarction |
| More information | CHMP summary of opinion for Eptifibatide Accord |
| Name of medicine | Lopinavir / Ritonavir Mylan |
|---|---|
| International non-proprietary name (INN) | lopinavir / ritonavir |
| Marketing-authorisation applicant | Mylan S.A.S. |
| Therapeutic indication | Treatment of HIV infection in adults, adolescents and children above the age of 2 years |
| More information | CHMP summary of opinion for Lopinavir / Ritonavir Mylan |
| Name of medicine | Pemetrexed Accord |
|---|---|
| International non-proprietary name (INN) | pemetrexed |
| Marketing-authorisation applicant | Accord Healthcare Ltd |
| Therapeutic indication | Treatment of unresectable malignant pleural mesothelioma and locally advanced or metastatic non-small cell lung cancer |
| More information | CHMP summary of opinion for Pemetrexed Accord |
| Name of medicine | Pemetrexed Actavis |
|---|---|
| International non-proprietary name (INN) | pemetrexed |
| Marketing-authorisation applicant | Actavis Group PTC ehf |
| Therapeutic indication | Treatment of unresectable malignant pleural mesothelioma and locally advanced or metastatic non-small cell lung cancer |
| More information | CHMP summary of opinion for Pemetrexed Actavis |
| Name of medicine | Solumarv |
|---|---|
| International non-proprietary name (INN) | insulin human |
| Marketing-authorisation applicant | Marvel Life Sciences Ltd |
| Therapeutic indication | Treatment of diabetes |
| More information | Questions and answers on refusal of the marketing authorisation for Solumarv (insulin human) |
| Name of medicine | Cimzia |
|---|---|
| INN | certolizumab pegol |
| Marketing-authorisation holder | UCB Pharma SA |
| More information | CHMP post-authorisation summary of positive opinion for Cimzia |
| Name of medicine | Pyramax |
|---|---|
| INN | pyronaridine-artesunate |
| Marketing-authorisation holder | Shin Poong Pharmaceutical Co., Ltd. |
| More information |
| Name of medicine | Zutectra |
|---|---|
| INN | human hepatitis b immunoglobulin |
| Marketing-authorisation holder | Biotest Pharma GmbH |
| More information | CHMP post-authorisation summary of positive opinion for Zutectra |
| Name of medicine | Article-20 procedure: Human papillomavirus (HPV) vaccines |
|---|---|
| More information | HPV vaccines: EMA confirms evidence does not support that they cause CRPS or POTS |