Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 17-20 November 2014
NewsHuman
Ten new medicines, two orphans, recommended for approval
Ten new medicines have been recommended for approval at the November 2014 meeting of the Committee for Medicinal Products for Human Use (CHMP).
The CHMP has recommended granting a marketing authorisation for Cerdelga (eliglustat), an orphan medicine for the treatment of Gaucher disease type 1. For more information please see the press release in the grid below.
The Committee also gave positive opinions for Exviera (dasabuvir) and Viekirax (ombitasvir + paritaprevir + ritonavir) for the treatment of chronic hepatitis C. Both products were reviewed under accelerated assessment and belong to a new generation of antiviral products for chronic hepatitis C infection that have high cure rates and have recently reshaped the treatment landscape for the disease. For more information please see the press release in the grid below.
The orphan medicine Ofev (nintedanib) received a positive opinion for the treatment of idiopathic pulmonary fibrosis.
Cosentyx (secukinumab) and Otezla (apremilast) were recommended by the CHMP as new treatment options for psoriasis.
Senshio (ospemifene) received a positive opinion for the treatment of vulvar and vaginal atrophy and Zontivity (vorapaxar) was recommended for the reduction of atherothrombotic events.
The CHMP also granted positive opinions for two new informed consent applications: Sevelamer carbonate Zentiva (sevelamer) for the control of hyperphosphataemia in adults receiving haemodialysis or peritoneal dialysis and Rasagiline ratiopharm (rasagiline) for the treatment of Parkinson's disease. An informed consent application is based on data from the dossier of a previously authorised medicine, with the marketing authorisation holder of that medicine giving consent for the use of their data in the application.
Two recommendations on extensions of therapeutic indications
The Committee recommended extensions of indications for Inductos and Travatan.
Change to non-prescription status
The CHMP recommended a change in classification status from prescription to non-prescription for the emergency contraceptive ellaOne (ulipristal acetate). For more information, please see the press release in the grid below.
Outcome of safety review
The CHMP concluded its review of Corlentor/Procoralan (ivabradine) and made recommendations aimed at reducing the risk of heart problems, including heart attack and bradycardia (excessively low heart rate), in patients taking the medicine for angina. Corlentor/Procoralan is used to treat symptoms of angina (chest pain due to problems with the blood flow to the heart) and to treat heart failure.
Update on Tecfidera
The CHMP discussed a fatal case of progressive multifocal leukoencephalopathy (PML) which was reported in a patient treated with Tecfidera (dimethyl fumarate), a medicine used for relapsing-remitting multiple sclerosis. This is the first case of PML, a rare viral brain infection with symptoms that can be similar to those of a multiple sclerosis attack, to be reported in association with Tecfidera. The fatal case of PML occurred after long-term treatment with the medicine in a patient experiencing severe long-term lymphopenia, a known possible side effect of Tecfidera.
The CHMP recommended that a letter is sent to healthcare professionals to alert them to the potential risk of PML and to allow them to inform their patients.
Withdrawals of applications
The application for a marketing authorisation for Egranli has been withdrawn. For more information, please see the question-and-answer document in the grid below.
An application for an extension of therapeutic indication for Ceprotin has been withdrawn. A question-and-answer document on the withdrawal is available below.
More information on other outcomes of the November 2014 CHMP meeting, is available in the grid below.
Agenda and minutes
The agenda of the November 2014 meeting is published on the EMA website. The minutes of the October meeting will be published during the week following the November CHMP meeting.
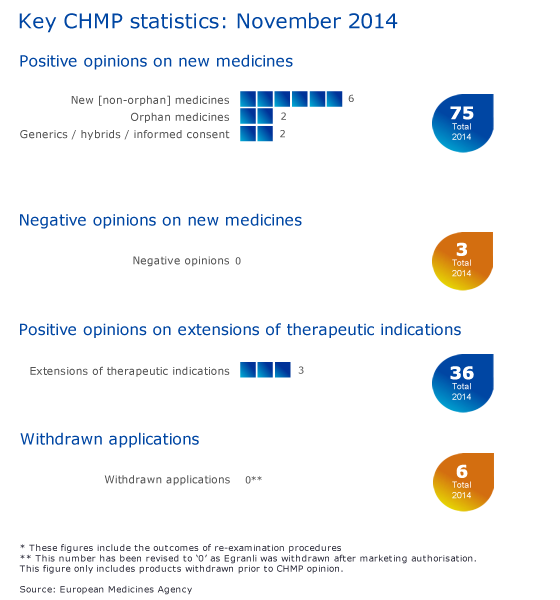
CHMP statistics
Key figures from the November 2014 CHMP meeting are represented in the graphic below.
| Name of medicine | Cerdelga |
|---|---|
| International non-proprietary name (INN) | eliglustat |
| Marketing-authorisation applicant | Genzyme Europe BV |
| Therapeutic indication | Treatment of Gaucher disease type 1 |
| More information |
CHMP summary of positive opinion for Cerdelga Press release: Cerdelga recommended for approval in type 1 Gaucher disease |
| Name of medicine | Cosentyx |
|---|---|
| INN | secukinumab |
| Marketing-authorisation applicant | Novartis Europharm Ltd |
| Therapeutic indication | Treatment of psoriasis |
| More information | CHMP summary of positive opinion for Cosentyx |
| Name of medicine | Exviera |
|---|---|
| INN | dasabuvir |
| Marketing-authorisation applicant | AbbVie Ltd |
| Therapeutic indication | Treatment of chronic hepatitis C |
| More information |
CHMP summary of positive opinion for Exviera Press release: Two new medicines recommended for the treatment of chronic hepatitis C |
| Name of medicine | Ofev |
|---|---|
| INN | nintedanib |
| Marketing-authorisation applicant | Boehringer Ingelheim International GmbH |
| Therapeutic indication | Treatment of idiopathic pulmonary fibrosis |
| More information | CHMP summary of positive opinion for Ofev |
| Name of medicine | Otezla |
|---|---|
| INN | apremilast |
| Marketing-authorisation applicant | Celgene Europe Limited |
| Therapeutic indication | Treatment of psoriasis |
| More information | CHMP summary of positive opinion for Otezla |
| Name of medicine | Senshio |
|---|---|
| INN | ospemifene |
| Marketing-authorisation applicant | Shionogi Limited |
| Therapeutic indication | Treatment of vulvar and vaginal atrophy |
| More information | CHMP summary of positive opinion for Senshio |
| Name of medicine | Viekirax |
|---|---|
| INN | ombitasvir / paritaprevir / ritonavir |
| Marketing-authorisation applicant | AbbVie Ltd |
| Therapeutic indication | Treatment of chronic hepatitis C |
| More information |
CHMP summary of positive opinion for Viekirax Press release: Two new medicines recommended for the treatment of chronic hepatitis C |
| Name of medicine | Zontivity |
|---|---|
| INN | vorapaxar |
| Marketing-authorisation applicant | Merck Sharp & Dohme Limited |
| Therapeutic indication | Reduction of atherothrombotic events |
| More information | CHMP summary of positive opinion for Zontivity |
| Name of medicine | Rasagiline ratiopharm |
|---|---|
| INN | rasagiline |
| Marketing-authorisation applicant | Teva B.V. |
| Therapeutic indication | Treatment of Parkinson's disease |
| More information | CHMP summary of positive opinion for Rasagiline ratiopharm |
| Name of medicine | Sevelamer carbonate Zentiva |
|---|---|
| INN | sevelamer |
| Marketing-authorisation applicant | Genzyme Europe BV |
| Therapeutic indication | Control of hyperphosphataemia in adult patients receiving haemodialysis or peritoneal dialysis |
| More information | CHMP summary of positive opinion for Sevelamer carbonate Zentiva |
| Name of medicine | Inductos |
|---|---|
| INN | dibotermin alfa |
| Marketing-authorisation holder | Medtronic BioPharma B.V. |
| More information | CHMP post-authorisation summary of positive opinion for Inductos |
| Name of medicine | Travatan |
|---|---|
| INN | travoprost |
| Marketing-authorisation holder | Alcon Laboratories (UK) Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Travatan |
| Name of medicine | Corlentor / Procoralan |
|---|---|
| INN | ivabradine hydrochloride |
| Marketing-authorisation holder | Les Laboratoires Servier |
| More information | Corlentor and Procoralan |
| Name of medicine | Nasonex |
|---|---|
| INN | mometasone |
| Marketing-authorisation holder | Merck Sharp & Dohme |
| More information | Nasonex |
| Name of medicine | Ceprotin |
|---|---|
| INN | human protein C |
| More information | Ceprotin |
| Name of medicine | Egranli |
|---|---|
| INN | balugrastim |
| More information | Egranli |
| Name of medicine | ellaOne |
|---|---|
| INN | ulipristal acetate |
| Marketing-authorisation holder | Laboratoire HRA Pharma SA |
| More information |
CHMP post-authorisation summary of positive opinion for ellaOne Press release: EMA recommends availability of ellaOne emergency contraceptive without prescription |