Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 17-20 September 2018
NewsHumanMedicines
The European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) elected Harald Enzmann as its new chair at its September 2018 meeting, for a three-year mandate starting on 21 September.
This week's meeting of the CHMP was the last plenary meeting chaired by Dr Tomas Salmonson, who retires as chair after serving for two three-year mandates, the maximum allowed by the legislation. Dr Salmonson is a senior scientific advisor at the Swedish Medical Products Agency, where he has worked since 1986. He has been a member of the CHMP for more than 18 years and has served as chair of the Committee since September 2012.
EMA would like to thank Dr Salmonson for his outstanding contribution to the work of the CHMP, which has enabled progress in key areas of public health. In 2016, he was closely involved in the launch of the PRIority MEdicines scheme (PRIME), which supports the development of promising new medicines in the European Union (EU). Under his leadership, the Committee improved its benefit-risk methodologies considering new scientific developments and technological advances and it broke new ground in its engagement with key stakeholders, such as international regulators, health technology assessment bodies and patients, who have started to participate in the Committee's decision-making on medicines. Over the six years at the helm of the CHMP, he has promoted EMA and the EU regulatory system as a whole as a hub of excellence for medicines regulation for the benefit of public health in the EU and around the world.
Thirteen medicines recommended for approval, including three orphans
The CHMP recommended thirteen medicines for approval, including three orphan medicines1.
The Committee recommended granting a marketing authorisation for the gene therapy Luxturna (voretigene neparvovec), for the treatment of adults and children with inherited retinal dystrophy caused by RPE65 gene mutations, a rare genetic disorder which causes vision loss and usually leads to blindness. Luxturna was designated as an orphan medicine during its development. For more information, please see the press release in the grid below.
The CHMP recommended granting a marketing authorisation for Emgality (galcanezumab), a monoclonal antibody for the prevention of migraine. Emgality belongs to a new class of medicines that work by blocking the activity of calcitonin gene-related peptide (CGRP), a molecule that is involved in migraine attacks. For more information, please see the press release in the grid below.
The CHMP recommended granting a marketing authorisation for a new antibiotic, Vabomere (meropenem trihydrate / vaborbactam), for the treatment of various severe infections in adults. The development of new and effective antibiotics is one of the most powerful tools to fight antimicrobial resistance. For more information, please see the press release in the grid below.
Two more orphan medicines received a positive opinion from the Committee: Jivi (damoctocog alfa pegol), for the treatment of haemophilia A (congenital factor VIII deficiency), and Poteligeo (mogamulizumab), for the treatment of mycosis fungoides or Sézary syndrome.
The CHMP recommended granting marketing authorisations for two cancer medicines: Alunbrig (brigatinib), for the treatment of anaplastic lymphoma kinase-positive advanced non-small cell lung cancer, and Apealea (paclitaxel), for the treatment of ovarian cancer.
Delstrigo (doravirine / lamivudine / tenofovir disoproxil) and Pifeltro (doravirine) received positive opinions for the treatment of HIV-1 infection.
Three biosimilar medicines intended to reduce the duration of neutropenia and the incidence of febrile neutropenia due to chemotherapy received a positive opinion from the Committee: Fulphila (pegfilgrastim), Pelmeg (pegfilgrastim) and Ziextenzo (pegfilgrastim).
The CHMP granted a positive opinion for Buvidal (buprenorphine), a hybrid medicine for the treatment of opioid dependence. Hybrid applications rely in part on the results of pre-clinical tests and clinical trials of a reference product and in part on new data.
Negative recommendation on a new medicine following re-examination
The applicant for Exondys (eteplirsen) requested a re-examination of the Committee's negative opinion for this medicine adopted at the May 2018 meeting. After considering the grounds for this request, the CHMP re-examined the initial opinion and confirmed its previous recommendation to refuse the granting of a marketing authorisation for this medicine.
For more information on this negative opinion, please see the question-and-answer document in the grid below.
Seven recommendations on extensions of therapeutic indication
The Committee recommended extensions of indication for Cabometyx, Elebrato Ellipta, Gilenya, RoActemra, Trelegy Ellipta, Venclyxto and Xtandi.
Start of re-examination of recommendations on extension of therapeutic indication
The applicants for Blincyto (blinatumomab), Opdivo (nivolumab) and Yervoy (ipilimumab) have requested re-examination of the Committee's negative opinions for these medicines adopted at the July 2018 meeting. The CHMP will now re-examine the opinions and issue final recommendations.
For more information on these negative opinions, please see the question-and-answer documents in the grid below.
Update on valsartan review
The CHMP is expanding its review of impurities in valsartan following the detection of very low levels of N-nitrosodiethylamine (NDEA) in another active substance, losartan, made by Hetero Labs in India. As a result of the detection of this impurity by German authorities, the review will now include medicines containing four other 'sartans', namely, candesartan, irbesartan, losartan and olmesartan. For more information, please see the public health communication in the grid below.
Withdrawals of applications
Applications for initial marketing authorisations for Entolimod TMC (entolimod) and Treprostinil SciPharm Sàrl (treprostinil) have been withdrawn. Entolimod TMC was intended to be used to reduce the risk of death following exposure to potentially lethal amounts of radiation. Treprostinil SciPharm Sàrl was intended to be used to treat chronic thromboembolic pulmonary hypertension.
Questions-and-answers documents on these withdrawals are available in the grid below.
Agenda and minutes
The agenda of the September 2018 meeting is published on EMA's website. Minutes of the July 2018 CHMP meeting will be published in the coming weeks.
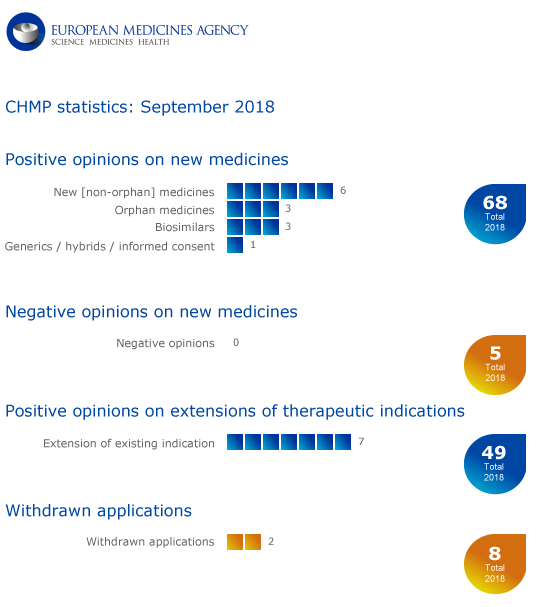
CHMP statistics
Key figures from the September 2018 CHMP meeting are represented in the graphic below.
1 As always at time of approval, these orphan designations will now be reviewed by EMA's Committee for Orphan Medicinal Products (COMP) to determine whether the information available to date allows maintaining the medicines' orphan status and granting the medicines ten years of market exclusivity.
CHMP statistics: September 2018
| Name of medicine | Alunbrig |
|---|---|
| International non-proprietary name (INN) | brigatinib |
| Marketing-authorisation applicant | Takeda Pharma A/S |
| Therapeutic indication | Treatment of adult patients with anaplastic lymphoma kinase positive advanced non-small cell lung cancer previously treated with crizotinib |
| More information | CHMP summary of positive opinion for Alunbrig |
| Name of medicine | Apealea |
|---|---|
| INN | paclitaxel |
| Marketing-authorisation applicant | Oasmia Pharmaceutical AB |
| Therapeutic indication | Treatment of ovarian cancer |
| More information | CHMP summary of positive opinion for Apealea |
| Name of medicine | Delstrigo |
|---|---|
| INN | doravirine / lamivudine / tenofovir disoproxil |
| Marketing-authorisation applicant | Merck Sharp & Dohme B.V. |
| Therapeutic indication | Treatment of HIV-1 infection |
| More information | CHMP summary of positive opinion for Delstrigo |
| Name of medicine | Emgality |
|---|---|
| INN | galcanezumab |
| Marketing-authorisation applicant | Eli Lilly Nederland B.V. |
| Therapeutic indication | Prophylaxis of migraine |
| More information |
Summary of opinion for Emgality
Press release: New medicine for the prevention of migraine |
| Name of medicine | Jivi |
|---|---|
| INN | damoctocog alfa pegol |
| Marketing-authorisation applicant | Bayer AG |
| Therapeutic indication | Treatment of haemophilia A (congenital factor VIII deficiency) |
| More information | CHMP summary of positive opinion for Jivi |
| Name of medicine | Luxturna |
|---|---|
| INN | voretigene neparvovec |
| Marketing-authorisation applicant | Spark Therapeutics Ireland Ltd |
| Therapeutic indication | Treatment of retinal dystrophies caused by RPE65 mutations |
| More information |
Press release: New gene therapy for rare inherited disorder causing vision loss recommended for approval |
| Name of medicine | Pifeltro |
|---|---|
| INN | doravirine |
| Marketing-authorisation applicant | Merck Sharp & Dohme B.V. |
| Therapeutic indication | Treatment of HIV-1 infection |
| More information | CHMP summary of positive opinion for Pifeltro |
| Name of medicine | Poteligeo |
|---|---|
| INN | mogamulizumab |
| Marketing-authorisation applicant | Kyowa Kirin Limited |
| Therapeutic indication | Treatment of mycosis fungoides or Sézary syndrome |
| More information | CHMP summary of positive opinion for Poteligeo |
| Name of medicine | Vabomere |
|---|---|
| INN | meropenem / vaborbactam |
| Marketing-authorisation applicant | Rempex London Ltd |
| Therapeutic indication |
Vabomere is indicated for the treatment of the following infections in adults:
Treatment of patients with bacteraemia that occurs in association with, or is suspected to be associated with, any of the infections listed above. Vabomere is also indicated for the treatment of infections due to aerobic Gram-negative organisms in adults with limited treatment options. |
| More information |
Summary of opinion for Vabomere
Press release: New medicine to treat infections in adults |
| Name of medicine | Fulphila |
|---|---|
| INN | pegfilgrastim |
| Marketing-authorisation applicant | MYLAN S.A.S |
| Therapeutic indication | To reduce the duration of neutropenia and the incidence of febrile neutropenia due to chemotherapy |
| More information | CHMP summary of positive opinion for Fulphila |
| Name of medicine | Pelmeg |
|---|---|
| INN | pegfilgrastim |
| Marketing-authorisation applicant | Cinfa Biotech S.L. |
| Therapeutic indication | To reduce the duration of neutropenia and the incidence of febrile neutropenia due to chemotherapy |
| More information | CHMP summary of positive opinion for Pelmeg |
| Name of medicine | Ziextenzo |
|---|---|
| INN | pegfilgrastim |
| Marketing-authorisation applicant | Sandoz GmbH |
| Therapeutic indication | To reduce the duration of neutropenia and the incidence of febrile neutropenia due to chemotherapy |
| More information | CHMP summary of positive opinion for Ziextenzo |
| Name of medicine | Buvidal |
|---|---|
| INN | buprenorphine |
| Marketing-authorisation applicant | Camurus AB |
| Therapeutic indication | Treatment of opioid dependence |
| More information | Summary of opinion for Buvidal |
| Name of medicine | Exondys |
|---|---|
| INN | eteplirsen |
| Marketing-authorisation applicant | AVI Biopharma International Ltd. |
| Therapeutic indication | Treatment of Duchenne muscular dystrophy |
| More information |
| Name of medicine | Cabometyx |
|---|---|
| INN | cabozantinib |
| Marketing-authorisation holder | Ipsen Pharma |
| More information | CHMP post-authorisation summary of positive opinion for Cabometyx |
| Name of medicine | Elebrato Ellipta |
|---|---|
| INN | fluticasone furoate / umeclidinium / vilanterol fluticasone |
| Marketing-authorisation holder | GlaxoSmithKline Trading Services Limited |
| More information | CHMP post-authorisation summary of positive opinion for Elebrato Ellipta |
| Name of medicine | Gilenya |
|---|---|
| INN | fingolimod |
| Marketing-authorisation holder | Novartis Europharm Limited |
| More information | CHMP post-authorisation summary of positive opinion for Gilenya (X/44/G) |
| Name of medicine | RoActemra |
|---|---|
| INN | tocilizumab |
| Marketing-authorisation holder | Roche Registration GmbH |
| More information | CHMP post-authorisation summary of positive opinion for RoActemra II-76 |
| Name of medicine | Trelegy Ellipta |
|---|---|
| INN | fluticasone furoate / umeclidinium / vilanterol fluticasone |
| Marketing-authorisation holder | GlaxoSmithKline Trading Services Limited |
| More information | CHMP post-authorisation summary of positive opinion for Trelegy Ellipta |
| Name of medicine | Venclyxto |
|---|---|
| INN | venetoclax |
| Marketing-authorisation holder | AbbVie Deutschland GmbH & Co. KG |
| More information | CHMP summary of positive opinion for Venclyxto (II/08) |
| Name of medicine | Xtandi |
|---|---|
| INN | enzalutamide |
| Marketing-authorisation holder | Astellas Pharma Europe B.V. |
| More information | CHMP post-authorisation summary of positive opinion for Xtandi II-39-G |
| Name of medicine | Blincyto |
|---|---|
| INN | blinatumomab |
| Marketing-authorisation applicant | Amgen Europe B.V. |
| More information | Questions and answers on Blincyto |
| Name of medicine | Opdivo |
|---|---|
| INN | nivolumab |
| Marketing-authorisation applicant | Bristol-Myers Squibb Pharma EEIG |
| More information | Questions and answers on Opdivo |
| Name of medicine | Yervoy |
|---|---|
| INN | ipilimumab |
| Marketing-authorisation applicant | Bristol-Myers Squibb Pharma EEIG |
| More information | Questions and answers on the refusal of a change to the marketing authorisations for Opdivo (nivolumab) and Yervoy (ipilimumab) |
| Name of medicine | Sartan medicines |
|---|---|
| INN | valsartan, candesartan, irbesartan, losartan and olmesartan |
| Marketing-authorisation holder | various companies |
| More information | Valsartan: review of impurities extended to other sartan medicines |
| Name of medicine | Entolimod TMC |
|---|---|
| INN | entolimod |
| Marketing-authorisation holder | TMC Pharma Services Ltd |
| More information |
| Name of medicine | Treprostinil SciPharm Sàrl |
|---|---|
| INN | treprostinil |
| Marketing-authorisation holder | SciPharm Sàrl |
| More information | Questions and answers on Treprostinil SciPharm Sàrl |