Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 18-21 April 2017
NewsHuman
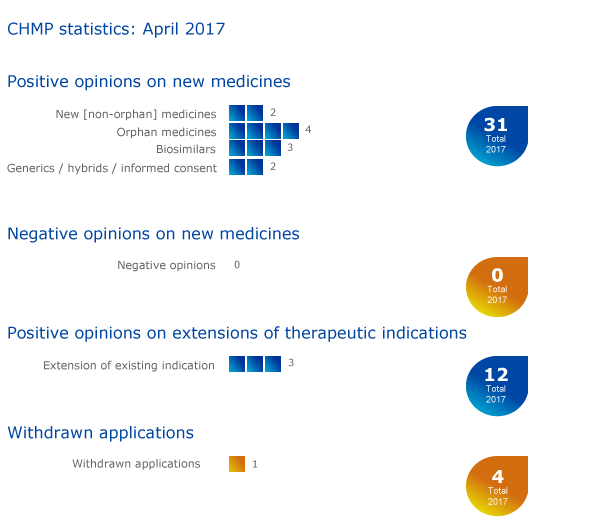
Eleven medicines recommended for approval, including four orphans
The European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) recommended eleven medicines for approval at its April meeting.
The CHMP recommended granting marketing authorisations for two orphan medicines to treat rare neurodegenerative conditions in children: Spinraza (nusinersen) to treat patients with spinal muscular atrophy (SMA) and Brineura (cerliponase alfa) to treat neuronal ceroid lipofuscinosis type 2 (CLN2) disease. Both medicines were reviewed under EMA's accelerated assessment programme. For more information on these medicines, please see the press releases in the grid below.
Besponsa (inotuzumab ozogamicin) received a positive opinion from the Committee for the treatment of acute lymphoblastic leukaemia. Besponsa has an orphan designation.
The CHMP granted a positive opinion for Kevzara (sarilumab) for the treatment of rheumatoid arthritis.
Skilarence (dimethyl fumarate) received a positive opinion from the Committee for the treatment of psoriasis.
One hybrid application, Cuprior (trientine tetrahydrochloride), received a positive opinion for the treatment of Wilson's disease, a rare autosomal recessive inherited disorder. Hybrid applications rely in part on the results of pre-clinical tests and clinical trials for a reference product and in part on new data. Cuprior has an orphan designation.
Three biosimilar medicines were recommended for approval by the Committee: Erelzi (etanercept) for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, axial spondyloarthritis, plaque psoriasis and paediatric plaque psoriasis; and Rixathon and Riximyo, both containing rituximab, for the treatment of non-Hodgkin's lymphoma, rheumatoid arthritis, granulomatosis with polyangiitis and microscopic polyangiitis. Rixathon is also intended for the treatment of chronic lymphocytic leukaemia. A biosimilar medicine is a biological medicine that is highly similar to another biological medicine that is already authorised for use.
Two generic medicines received a positive opinion from the CHMP: Febuxostat Mylan (febuxostat) for the prevention and treatment of hyperuricaemia and Ucedane (carglumic acid) for the treatment of hyperammonaemia due to N-acetylglutamate synthase primary deficiency.
Three recommendations on extensions of therapeutic indications
The Committee recommended extensions of indications for Avastin, Celsentri and Opdivo.
Withdrawal of application
An application for an initial marketing authorisation for Solithromycin Triskel EU Services (solithromycin) has been withdrawn. This medicine was intended for the treatment of community-acquired pneumonia, inhaled anthrax and inhaled tularaemia. A questions-and-answers document on this withdrawal is available in the grid below.
Agenda and minutes
The agenda of the April 2017 meeting is published on EMA's website. Minutes of the March 2017 CHMP meeting will be published next week.
CHMP statistics
Key figures from the April 2017 CHMP meeting are represented in the graphic below.
More information on all other outcomes of the CHMP's April 2017 meeting is available in the grid below.
| Name of medicine | Besponsa |
|---|---|
| International non-proprietary name (INN) | inotuzumab ozogamicin |
| Marketing-authorisation applicant | Pfizer Limited |
| Therapeutic indication | Treatment of acute lymphoblastic leukaemia |
| More information | CHMP summary of positive opinion for Besponsa |
| Name of medicine | Brineura |
|---|---|
| INN | cerliponase alfa |
| Marketing-authorisation applicant | BioMarin International Limited |
| Therapeutic indication | Treatment of neuronal ceroid lipofuscinosis type 2 (CLN2) disease |
| More information |
CHMP summary of positive opinion for Brineura
Press release: New medicine for rare neurodegenerative disorder in children |
| Name of medicine | Cuprior |
|---|---|
| INN | trientine tetrahydrochloride |
| Marketing-authorisation applicant | GMP-Orphan SA |
| Therapeutic indication | Treatment of Wilson's disease |
| More information | CHMP summary of positive opinion for Cuprior |
| Name of medicine | Kevzara |
|---|---|
| INN | sarilumab |
| Marketing-authorisation applicant | Sanofi-Aventis groupe |
| Therapeutic indication | Treatment of rheumatoid arthritis |
| More information | CHMP summary of positive opinion for Kevzara |
| Name of medicine | Skilarence |
|---|---|
| INN | dimethyl fumarate |
| Marketing-authorisation applicant | Almirall S.A |
| Therapeutic indication | Treatment of psoriasis |
| More information | CHMP summary of positive opinion for Skilarence |
| Name of medicine | Spinraza |
|---|---|
| INN | nusinersen |
| Marketing-authorisation applicant | Biogen Idec Ltd |
| Therapeutic indication | Treatment of spinal muscular atrophy |
| More information |
CHMP summary of positive opinion for Spinraza
Press release: First medicine for spinal muscular atrophy |
| Name of medicine | Febuxostat Mylan |
|---|---|
| INN | febuxostat |
| Marketing-authorisation applicant | MYLAN S.A.S |
| Therapeutic indication | Prevention and treatment of hyperuricaemia |
| More information | CHMP summary of positive opinion for Febuxostat Mylan |
| Name of medicine | Ucedane |
|---|---|
| INN | carglumic acid |
| Marketing-authorisation applicant | Lucane Pharma - Paris |
| Therapeutic indication | Treatment of hyperammonaemia due to N-acetylglutamate synthase primary deficiency |
| More information | CHMP summary of positive opinion for Ucedane |
| Name of medicine | Erelzi |
|---|---|
| INN | etanercept |
| Marketing-authorisation applicant | Sandoz GmbH |
| Therapeutic indication | Treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, axial spondyloarthritis, plaque psoriasis and paediatric plaque psoriasis |
| More information | CHMP summary of positive opinion for Erelzi |
| Name of medicine | Rixathon |
|---|---|
| INN | rituximab |
| Marketing-authorisation applicant | Sandoz GmbH |
| Therapeutic indication | Treatment of non-Hodgkin's lymphoma, chronic lymphocytic leukaemia, rheumatoid arthritis, granulomatosis with polyangiitis and microscopic polyangiitis |
| More information | CHMP summary of positive opinion for Rixathon |
| Name of medicine | Riximyo |
|---|---|
| INN | rituximab |
| Marketing-authorisation applicant | Sandoz GmbH |
| Therapeutic indication | Treatment of non-Hodgkin's lymphoma, rheumatoid arthritis, granulomatosis with polyangiitis and microscopic polyangiitis |
| More information | CHMP summary of positive opinion for Riximyo |
| Name of medicine | Avastin |
|---|---|
| INN | bevacizumab |
| Marketing-authorisation holder | Roche Registration Limited |
| More information | CHMP post-authorisation summary of positive opinion for Avastin (II/0092) |
| Name of medicine | Celsentri |
|---|---|
| INN | maraviroc |
| Marketing-authorisation holder | ViiV Healthcare UK Limited |
| More information | CHMP post-authorisation summary of positive opinion for Celsentri (X-46-G) |
| Name of medicine | Opdivo |
|---|---|
| INN | nivolumab |
| Marketing-authorisation holder | Bristol-Myers Squibb Pharma EEIG |
| More information | CHMP post-authorisation summary of positive opinion for Opdivo |
| Name of medicine | Etopophos and associated names |
|---|---|
| INN | etoposide |
| Marketing-authorisation holder | Bristol-Myers Squibb group of companies and associated companies |
| More information | Questions and answers on Etopophos and associated names |
| Name of medicine | Vepesid and associated names |
|---|---|
| INN | etoposide |
| Marketing-authorisation holder | Bristol-Myers Squibb group of companies and associated companies |
| More information | Questions and answers on Vepesid and associated names |
| Name of medicine | Solithromycin Triskel EU Services |
|---|---|
| INN | solithromycin |
| More information | Questions and answers on Solithromycin Triskel EU Services |