
Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 22-25 July 2024
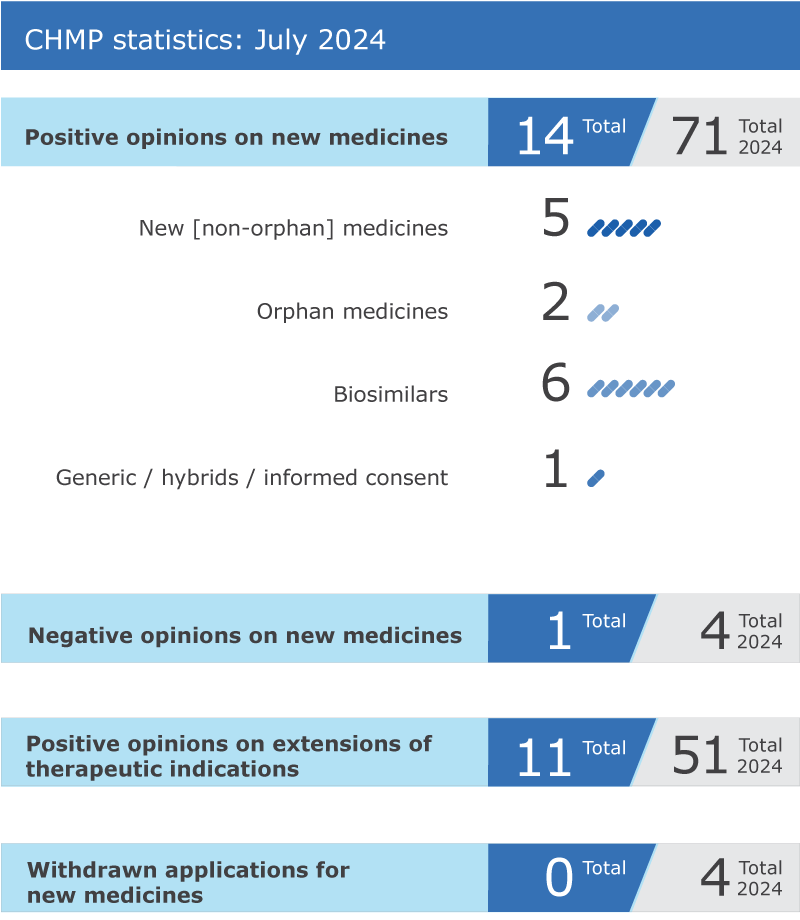
14 new medicines recommended for approval; another 11 medicines recommended for extension of therapeutic indications
NewsHumanMedicines
EMA’s human medicines committee (CHMP) recommended 14 medicines for approval at its July 2024 meeting.
The CHMP recommended granting a marketing authorisation for Anzupgo (delgocitinib), a medicine intended for the treatment of moderate to severe chronic hand eczema in adults for whom topical corticosteroids are inadequate or inappropriate.
The committee recommended granting a conditional marketing authorisation for Iqirvo* (elafibranor), for the treatment of primary biliary cholangitis, a chronic and progressive autoimmune disease that can cause liver damage.
Kayfanda (odevixibat), received a positive opinion under exceptional circumstances for the treatment of cholestatic pruritus in patients with Alagille syndrome, a rare, life-threatening genetic disorder with a wide variety of clinical manifestations affecting the liver, heart, skeleton, eyes, skin, central nervous system, kidneys, and facial features.
The CHMP adopted a positive opinion for Loqtorzi (toripalimab), for the treatment of nasopharyngeal carcinoma and oesophageal squamous cell carcinoma.
Vevizye (ciclosporin), received a positive opinion from the CHMP for the treatment of adult patients with moderate to severe dry eye disease which has not improved despite treatment with tear substitutes.
The CHMP recommended granting a marketing authorisation for Vyloy* (zolbetuximab), to treat gastric or gastro-oesophageal junction adenocarcinoma, a cancer of the stomach.
A positive opinion was adopted for Yuvanci (macitentan / tadalafil), for the treatment of pulmonary arterial hypertension, a chronic and progressive disease of the small pulmonary arteries that is characterised by vascular proliferation and remodelling.
The committee adopted positive opinions for six biosimilar medicines:
The committee also recommended granting a marketing authorisation for Axitinib Accord (axitinib), a generic medicine for the treatment of adult patients with advanced renal cell carcinoma.
The CHMP recommended not granting a marketing authorisation for Leqembi (lecanemab), a medicine intended for the treatment of Alzheimer’s disease. The committee considered that the observed effect of Leqembi on delaying cognitive decline does not counterbalance the risk of serious side events associated with the medicine, in particular the frequent occurrence of amyloid-related imaging abnormalities (ARIA), involving swelling and potential bleedings in the brain of patients who received Leqembi.
For more information on this negative opinion, see the question-and-answer document in the grid below.
The committee recommended extensions of indication for 11 medicines that are already authorised in the EU: Arexvy, Braftovi, Edurant, Keytruda, Mektovi, Opsumit, Padcev, Rybrevant, Slenyto, Spevigo and Tecentriq.
The marketing authorisation holders for Masitinib AB Science* (masitinib), Syfovre (pegcetacoplan) and Translarna* (ataluren) have requested a re-examination of the opinions adopted during the committee’s June 2024 meeting. Upon receipt of the grounds of the requests, the CHMP will re-examine its opinions and issue final recommendations.
The CHMP has recommended strengthening existing advice to minimise the risks from interactions between the weight loss medicine Mysimba (naltrexone / bupropion) and opioid-containing medicines, such as the opioid painkillers morphine and codeine, other opioids used during surgery, and certain medicines for cough, cold or diarrhoea. For more information, see the public health communication in the grid below.
The CHMP has finalised its assessment of an application to extend the use of the weight loss medicine Wegovy (semaglutide) to include prevention of major cardiovascular problems in adults with established cardiovascular disease and a body mass index (BMI) of at least 27 kg/m2. The CHMP considered that this use is already covered by the approved indication for weight management and therefore did not agree to add a separate indication for the prevention of cardiovascular disease. Instead, it recommended to include additional information from a study in the product information. For more information on the CHMP opinion for this medicine, see the question-and-answer document in the grid below.
The agenda of the July 2024 CHMP meeting is published on EMA's website. Minutes of the meeting will be published in the coming weeks.
Key figures from the July 2024 CHMP meeting are represented in the graphic below.
*This product was designated as an orphan medicine during its development. Orphan designations are reviewed by EMA's Committee for Orphan Medicinal Products (COMP) at the time of approval to determine whether the information available to date allows maintaining the medicine’s orphan status and granting the medicine ten years of market exclusivity.
delgocitinib
LEO Pharma A/S
Treatment of moderate to severe chronic hand eczema (CHE)
elafibranor
Ipsen Pharma
Treatment of primary biliary cholangitis (PBC)
odevixibat
Ipsen Pharma
Treatment of cholestatic pruritus in Alagille syndrome (ALGS)
toripalimab
TMC Pharma (EU) Limited
Combination treatment for metastatic or recurrent locally advanced nasopharyngeal carcinoma and for metastatic or recurrent oesophageal squamous cell carcinoma
ciclosporin
Novaliq GmbH
Treatment of dry eye disease in adult patients
zolbetuximab
Astellas Pharma Europe B.V.
Treatment of locally advanced unresectable or metastatic HER2 negative gastric or gastro-oesophageal junction (GEJ) adenocarcinoma
macitentan / tadalafil
Janssen-Cilag International NV
Treatment of pulmonary arterial hypertension (PAH) in adults
ustekinumab
Samsung Bioepis NL B.V.
Treatment of Crohn’s disease, Ulcerative colitis, Plaque psoriasis, Paediatric plaque psoriasis and Psoriatic arthritis (PsA)
ustekinumab
Formycon AG
Treatment of Crohn’s disease, Ulcerative colitis, Plaque psoriasis, Paediatric plaque psoriasis and Psoriatic arthritis (PsA)
rituximab
Reddy Holding GmbH
Treatment of Non-Hodgkin's lymphoma (NHL), Chronic lymphocytic leukaemia (CLL) and Rheumatoid arthritis
ustekinumab
Fresenius Kabi Deutschland GmbH
Treatment of Crohn’s disease, Plaque psoriasis, Paediatric plaque psoriasis and Psoriatic arthritis (PsA)
ranibizumab
MIDAS Pharma GmbH
Treatment of neovascular (wet) age-related macular degeneration (AMD), visual impairment due to diabetic macular oedema (DME), proliferative diabetic retinopathy (PDR), visual impairment due to macular oedema secondary to retinal vein occlusion (branch RVO or central RVO) and visual impairment due to choroidal neovascularisation (CNV)
trastuzumab
Prestige Biopharma Belgium
Indicated for the treatment of adult patients with HER2 positive metastatic breast cancer (MBC) and HER2 positive early breast cancer (EBC)
axitinib
Accord Healthcare S.L.U.
Treatment of adult patients with advanced renal cell carcinoma (RCC)
lecanemab
Eisai GmbH
A disease modifying treatment in adult patients with Mild Cognitive Impairment due to Alzheimer’s disease and Mild Alzheimer’s disease (Early Alzheimer’s disease)
Respiratory syncytial virus, glycoprotein F, recombinant, stabilised in the pre-fusion conformation, adjuvanted with AS01E
GlaxoSmithkline Biologicals S.A.
encorafenib
Pierre Fabre Medicament
rilpivirine
Janssen-Cilag International N.V.
binimetinib
Pierre Fabre Medicament
pembrolizumab
Merck Sharp & Dohme B.V.
macitentan
Janssen-Cilag International N.V.
enfortumab vedotin
Astellas Pharma Europe B.V.
amivantamab
Janssen-Cilag International N.V.
melatonin
RAD Neurim Pharmaceuticals EEC SARL
spesolimab
Boehringer Ingelheim International GmbH
atezolizumab
Roche Registration GmbH
masitinib
AB Science
In combination with riluzole for the treatment of adult patients with amyotrophic lateral sclerosis (ALS)
pegcetacoplan
Apellis Europe B.V.
Treatment of geographic atrophy (GA) secondary to age-related macular degeneration (AMD)
ataluren
PTC Therapeutics International Limited