Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 28-31 May 2018
NewsHuman
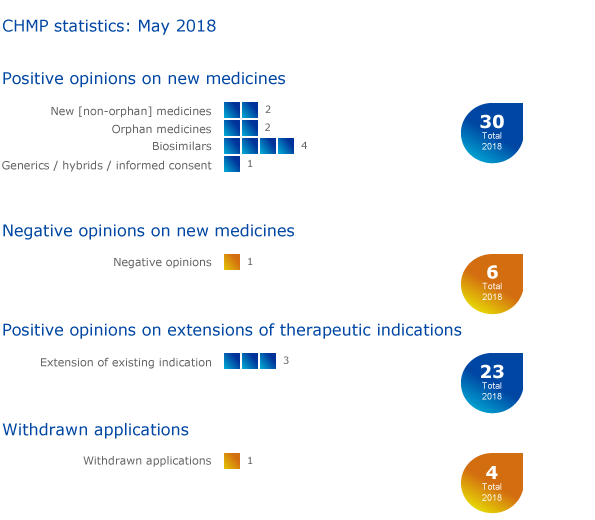
The European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) recommended nine medicines for approval, including two orphan medicines1, at its May 2018 meeting.
The CHMP recommended granting a marketing authorisation for Aimovig (erenumab), the first human monoclonal antibody therapy for prevention of migraine. Aimovig belongs to a new class of medicines that work by blocking the activity of calcitonin gene-related peptide, a molecule that is involved in migraine attacks. For more information, please see the press release in the grid below.
The Committee recommended granting a marketing authorisation for Tegsedi (inotersen), a medicine for patients with hereditary transthyretin amyloidosis that aims to affect the course of the disease and improve quality of life. This medicine was reviewed under EMA's accelerated assessment mechanism, reserved for medicines of major public health interest. Tegsedi was designated as an orphan medicine during its development. For more information, please see the press release in the grid below.
Myalepta (metreleptin) received a positive opinion for the treatment of leptin deficiency. Myalepta was designated as an orphan medicine during its development.
The CHMP recommended granting a marketing authorisation for Rxulti (brexpiprazole), for the treatment of schizophrenia.
Four biosimilar medicines received a positive opinion from the Committee: Halimatoz, Hefiya and Hyrimoz, all containing adalimumab, were recommended for the treatment of certain inflammatory and autoimmune disorders; and Trazimera (trastuzumab), was recommended for the treatment of breast and gastric cancer.
The Committee recommended for approval the generic medicine Nityr (nitisinone), for the treatment of hereditary tyrosinemia type 1.
Negative opinion on new medicine
The CHMP adopted a negative opinion refusing a marketing authorisation for Exondys (eteplirsen). Exondys was expected to be used to treat Duchenne muscular dystrophy. The applicant requested a re-examination of this negative opinion on 1 June 2018. For more information please see the question-and-answer document in the grid below.
Update on re-examination
The applicant for Alsitek (masitinib) has withdrawn the request for re-examination of the Committee's negative opinion for this medicine. For more information please see the question-and-answer document.
Three recommendations on extensions of therapeutic indication
The Committee recommended extensions of indications for Briviact, Translarna and Xeljanz.
Start of referral: metamizole-containing medicines
The Committee also started a review of medicines containing the painkiller metamizole. There are substantial differences between member states in the recommended maximum daily doses of the medicine and the contraindications on its use during pregnancy or in women who are breast feeding. For more information please see the start-of-referral document in the grid below.
Outcome of review on Esmya
The CHMP recommended several measures to minimise the risk of rare but serious liver injury with Esmya (ulipristal acetate), for the treatment of moderate to severe symptoms of uterine fibroids. For more information please see the public health recommendation in the grid below.
Outcome of review on Keytruda and Tecentriq
The CHMP recommended restricting the use of Keytruda (pembrolizumab) and Tecentriq (atezolizumab) as first line-treatments for urothelial cancer, because new data indicate that these medicines may not work as well as chemotherapy in some patients with low levels of the protein PD-L1. For more information please see the public health recommendation in the grid below.
Withdrawal of application
The application for an initial marketing authorisation for Restaysis (ciclosporin) was withdrawn. This medicine was intended to be used for the treatment of moderate dry eye disease. A question-and-answer document on this withdrawal is available in the grid below.
Agenda and minutes
The agenda of the May 2018 meeting is published on EMA's website. Minutes of the April 2018 CHMP meeting will be published in the coming weeks.
CHMP statistics
Key figures from the May 2018 CHMP meeting are represented in the graphic below.
More information on all other outcomes of the CHMP May 2018 meeting is available in the grid below.
1 As always at time of approval, these orphan designations will now be reviewed by EMA's Committee for Orphan Medicinal Products (COMP) to determine whether the information available to date allows maintaining the medicines' orphan status and granting the medicines ten years of market exclusivity.
| Name of medicine | Aimovig |
|---|---|
| International non-proprietary name (INN) | erenumab |
| Marketing-authorisation applicant | Novartis Europharm Limited |
| Therapeutic indication | Prophylaxis of migraine |
| More information |
CHMP summary of positive opinion for Aimovig
Press release: First monoclonal antibody therapy for prevention of migraine |
| Name of medicine | Myalepta |
|---|---|
| INN | metreleptin |
| Marketing-authorisation applicant | Aegerion Pharmaceuticals B.V. |
| Therapeutic indication | Treatment of leptin deficiency (lipodystrophy) |
| More information | CHMP summary of positive opinion for Myalepta |
| Name of medicine | Rxulti |
|---|---|
| INN | brexpiprazole |
| Marketing-authorisation applicant | Otsuka Pharmaceutical Europe Ltd |
| Therapeutic indication | Treatment of schizophrenia |
| More information | CHMP summary of positive opinion for Rxulti |
| Name of medicine | Tegsedi |
|---|---|
| INN | inotersen |
| Marketing-authorisation applicant | IONIS USA Ltd |
| Therapeutic indication | Treatment of hereditary transthyretin amyloidosis |
| More information |
CHMP summary of positive opinion for Tegsedi
Press release: New medicine for hereditary rare disease |
| Name of medicine | Nityr |
|---|---|
| International non-proprietary name (INN) | nitisinone |
| Marketing-authorisation applicant | Cycle Pharmaceuticals Ltd |
| Therapeutic indication | Treatment of hereditary tyrosinemia type 1 |
| More information | CHMP summary of positive opinion for Nityr |
| Name of medicine | Halimatoz |
|---|---|
| International non-proprietary name (INN) | adalimumab |
| Marketing-authorisation applicant | Sandoz GmbH |
| Therapeutic indication | Treatment of certain inflammatory and autoimmune disorders |
| More information | CHMP summary of positive opinion for Halimatoz |
| Name of medicine | Hefiya |
|---|---|
| INN | adalimumab |
| Marketing-authorisation applicant | Sandoz GmbH |
| Therapeutic indication | Treatment of certain inflammatory and autoimmune disorders |
| More information | CHMP summary of positive opinion for Hefiya |
| Name of medicine | Hyrimoz |
|---|---|
| INN | adalimumab |
| Marketing-authorisation applicant | Sandoz GmbH |
| Therapeutic indication | Treatment of certain inflammatory and autoimmune disorders |
| More information | CHMP summary of positive opinion for Hyrimoz |
| Name of medicine | Trazimera |
|---|---|
| INN | trastuzumab |
| Marketing-authorisation applicant | Pfizer Europe MA EEIG |
| Therapeutic indication | Treatment of breast and gastric cancer |
| More information | CHMP summary of positive opinion for Trazimera |
| Name of medicine | Exondys |
|---|---|
| INN | eteplirsen |
| Marketing-authorisation applicant | AVI Biopharma International Ltd |
| Therapeutic indication | Treatment of Duchenne muscular dystrophy |
| More information |
| Name of medicine | Briviact |
|---|---|
| INN | brivaracetam |
| Marketing-authorisation holder | UCB Pharma S.A. |
| More information | CHMP post-authorisation summary of positive opinion for Briviact |
| Name of medicine | Translarna |
|---|---|
| INN | ataluren |
| Marketing-authorisation holder | PTC Therapeutics International Limited |
| More information | CHMP post-authorisation summary of positive opinion for Translarna (II-37) |
| Name of medicine | Xeljanz |
|---|---|
| INN | tofacitinib |
| Marketing-authorisation holder | Pfizer Limited |
| More information | CHMP post-authorisation summary of positive opinion for Xeljanz (X-05-G) |
| Name of medicine | Metamizole containing medicinal products |
|---|---|
| INN | metamizole sodium |
| More information | EMA begins review of medicines containing metamizole |
| Name of medicine | Esmya |
|---|---|
| INN | ulipristal acetate |
| Marketing-authorisation holder | Gedeon Richter Plc |
| More information | Esmya: new measures to minimise risk of rare but serious liver injury |
| Name of medicine | Keytruda and Tecentriq |
|---|---|
| More information | EMA restricts use of Keytruda and Tecentriq in bladder cancer |
| Name of medicine | Scandonest and associated names |
|---|---|
| INN | mepivacaine |
| Marketing-authorisation holder | Septodont group of companies and associated companies |
| More information | Questions and answers on Scandonest and associated names |
| Name of medicine | Restaysis |
|---|---|
| INN | ciclosporin |
| Marketing-authorisation applicant | Allergan Pharmaceuticals International Limited |
| More information | Restaysis: Withdrawn application |