Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 6-9 November 2023
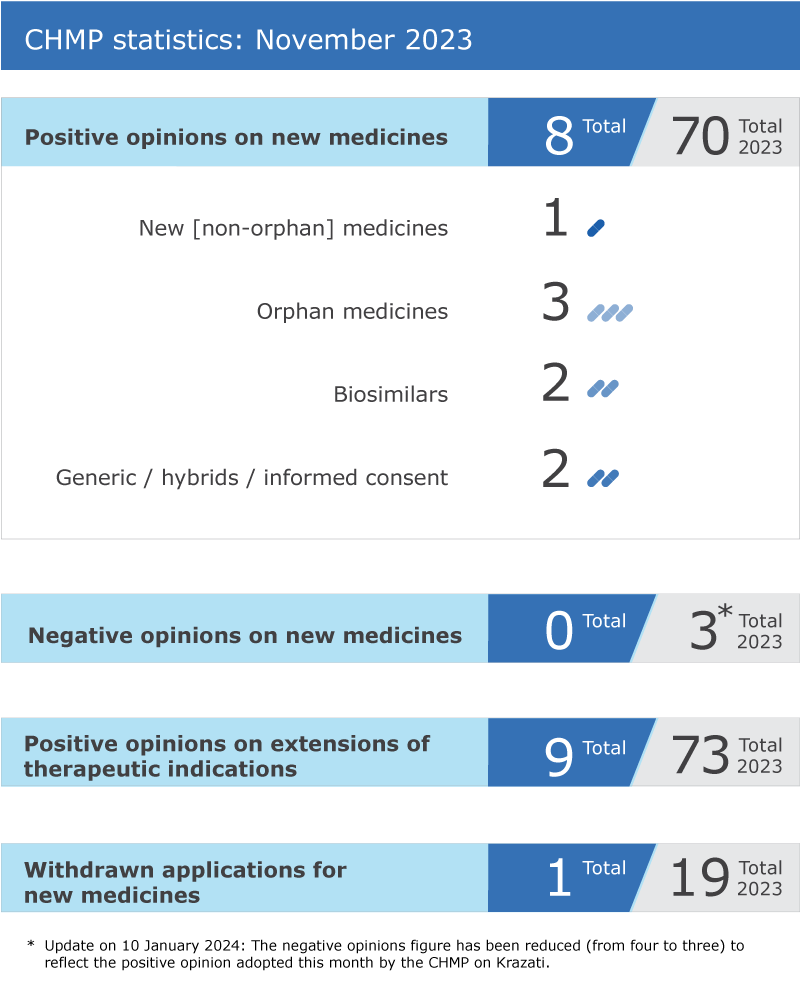
EMA’s human medicines committee (CHMP) recommended eight medicines.
NewsHumanMedicinesReferrals
EMA’s human medicines committee (CHMP) recommended eight medicines for approval at its November 2023 meeting.
The CHMP adopted a positive opinion for Omjjara* (momelotinib), for the treatment of myelofibrosis, a rare blood cancer that affects the bone marrow.
Rystiggo* (rozanolixizumab), intended for the treatment of generalised myasthenia gravis, received a positive opinion. Myasthenia gravis is a chronic autoimmune neuromuscular condition that causes muscle weakness in different parts of the body.
A positive opinion was adopted for Spexotras* (trametinib) for the treatment of paediatric patients aged one year and older with glioma, a type of brain tumour that begins in glial cells (the cells that surround and support nerve cells).
The committee adopted positive opinions for two biosimilar medicines: Rimmyrah (ranibizumab), for the treatment of neovascular age-related macular degeneration, a progressive retinal macular disease causing gradual vision impairment mainly in the elderly; and Uzpruvo (ustekinumab), for the treatment of plaque psoriasis, psoriatic arthritis and Crohn’s disease.
Two generic medicines also received a positive opinion from the committee: Azacitidine Kabi (azacitidine), for the treatment of myelodysplastic syndromes, chronic myelomonocytic leukaemia and acute myeloid leukaemia; and Naveruclif (paclitaxel), for the treatment of metastatic breast cancer, metastatic adenocarcinoma of the pancreas and non-small cell lung cancer.
Following a re-examination, the CHMP recommended granting a conditional marketing authorisation for Krazati (adagrasib), for the treatment of adults with advanced non-small cell lung cancer with a G12C mutation in the KRAS gene whose disease has worsened after at least one systemic treatment.
A question-and-answer document is available in the grid below.
The committee recommended extensions of indication for nine medicines that are already authorised in the EU: Ayvakyt*, Evkeeza, Fluad Tetra, Jardiance, Keytruda, Mounjaro, NexoBrid, Talzenna, Veltassa.
One application for an initial marketing authorisation was withdrawn. Vijoice* was intended for the treatment of PIK3CA-related overgrowth spectrum, a genetic condition that causes a range of symptoms including malformations and abnormal growth or tumours affecting several tissues, such as the skin, bones, blood vessels and brain.
The marketing authorisation holder for Bylvay* withdrew an application to extend the use of this medicine to include the treatment of cholestatic pruritus in Alagille syndrome in patients aged six months or older.
Question-and-answer documents on these withdrawals are available in the grid below.
The CHMP started a review of antibiotic medicines containing azithromycin that are given by mouth or by injection. The review has been initiated at the request of the German medicines regulatory agency under Article 31 of Directive 2001/83/EC.
For more information, see the public health communication in the grid below.
The CHMP agreed to update the product information for the anticoagulant medicine Pradaxa to remove a pharmaceutical form (powder and solvent for oral solution) and to change the existing indication for use in children under 18 years of age.
The agenda of the November 2023 CHMP meeting is published on EMA's website. Minutes of the meeting will be published in the coming weeks.
Key figures from the November 2023 CHMP meeting are represented in the graphic below.
*This product was designated as an orphan medicine during its development. Orphan designations are reviewed by EMA's Committee for Orphan Medicinal Products (COMP) at the time of approval to determine whether the information available to date allows maintaining the medicine’s orphan status and granting the medicine ten years of market exclusivity.
| Name of medicine | Omjjara |
|---|---|
| INN | momelotinib |
| Marketing-authorisation applicant | Glaxosmithkline Trading Services Limited |
| Therapeutic indication | Omjjara is indicated for the treatment of disease-related splenomegaly or symptoms in adult patients with moderate to severe anaemia who have primary myelofibrosis, post polycythaemia vera myelofibrosis or post essential thrombocythaemia myelofibrosis and who are Janus Kinase (JAK) inhibitor naïve or have been treated with ruxolitinib. |
| More information | Omjjara: Pending EC decision |
| Name of medicine | Rystiggo |
|---|---|
| INN | rozanolixizumab |
| Marketing-authorisation applicant | UCB Pharma |
| Therapeutic indication | Treatment of generalised myasthenia gravis (gMG) |
| More information | Rystiggo: Pending EC decision |
| Name of medicine | Spexotras |
|---|---|
| INN | trametinib |
| Marketing-authorisation applicant | Novartis Europharm Limited; |
| Therapeutic indication | Treatment of paediatric patients aged 1 year and older with glioma |
| More information | Spexotras: Pending EC decision |
| Name of medicine | Rimmyrah |
|---|---|
| INN | ranibizumab |
| Marketing-authorisation applicant | QILU PHARMA SPAIN S.L. |
| Therapeutic indication | Treatment of neovascular age-related macular degeneration (AMD) |
| More information | Rimmyrah: Pending EC decision |
| Name of medicine | Uzpruvo |
|---|---|
| INN | ustekinumab |
| Marketing-authorisation applicant | STADA Arzneimittel AG |
| Therapeutic indication | Treatment of plaque psoriasis, arthritis psoriatic and Crohn’s Disease |
| More information | Uzpruvo: Pending EC decision |
| Name of medicine | Azacitidine Kabi |
|---|---|
| International non-proprietary name (INN) | azacitidine |
| Marketing-authorisation applicant | Fresenius Kabi Deutschland GmbH |
| Therapeutic indication | Treatment of myelodysplastic syndromes (MDS), chronic myelomonocytic leukaemia (CMML) and acute myeloid leukaemia (AML) |
| More information | Azacitidine Kabi: Pending EC decision |
| Name of medicine | Naveruclif |
|---|---|
| INN | paclitaxel |
| Marketing-authorisation applicant | Accord Healthcare S.L.U. |
| Therapeutic indication | Treatment of metastatic breast cancer |
| More information | Naveruclif: Pending EC decision |
| Name of medicine | Krazati |
|---|---|
| INN | adagrasib |
| Marketing-authorisation applicant | Mirati Therapeutics B.V. |
| More information | Krazati: Pending EC decision |
| Name of medicine | Vijoice |
|---|---|
| INN | Alpelisib |
| Marketing-authorisation applicant | Novartis EuropharmLimited |
| More information | Vijoice: Withdrawn application |
| Name of medicine | Ayvakyt |
|---|---|
| INN | avapritinib |
| Marketing-authorisation holder | Blueprint Medicines (Netherlands) B.V. |
| More information | Ayvakyt: Pending EC decision |
| Name of medicine | Evkeeza |
|---|---|
| INN | evinacumab |
| Marketing-authorisation holder | Ultragenyx Germany GmbH |
| More information | Evkeeza: Pending EC decision |
| Name of medicine | Fluad Tetra |
|---|---|
| COMMON NAME | influenza vaccine (surface antigen, inactivated, adjuvanted) |
| Marketing-authorisation holder | Seqirus Netherlands B.V. |
| More information | Fluad Tetra: Pending EC decision |
| Name of medicine | Jardiance |
|---|---|
| INN | empagliflozin |
| Marketing-authorisation holder | Boehringer Ingelheim International GmbH |
| More information | Jardiance: Pending EC decision |
| Name of medicine | Keytruda |
|---|---|
| INN | pembrolizumab |
| Marketing-authorisation holder | Merck Sharp & Dohme B.V. |
| More information | Keytruda: Pending EC decision |
| Name of medicine | Mounjaro |
|---|---|
| INN | tirzepatide |
| Marketing-authorisation holder | Eli Lilly Nederland B.V. |
| More information | Mounjaro: Pending EC decision |
| Name of medicine | NexoBrid |
|---|---|
| INN | concentrate of proteolytic enzymes enriched in bromelain |
| Marketing-authorisation applicant | MediWound Germany GmbH |
| More information | NexoBrid: Pending EC decision |
| Name of medicine | Talzenna |
|---|---|
| INN | talazoparib |
| Marketing-authorisation holder | Pfizer Europe MA EEIG |
| More information | Talzenna: Pending EC decision |
| Name of medicine | Veltassa |
|---|---|
| INN | patiromer |
| Marketing-authorisation holder | Vifor Fresenius Medical Care Renal Pharma France |
| More information | Veltassa: Pending EC decision |
| Name of medicine | Bylvay |
|---|---|
| INN | odevixibat |
| Marketing-authorisation holder | Albireo |
| More information | Bylvay: Withdrawn application |
| Name of medicine | Azithromycin-containing medicinal products for systemic use |
|---|---|
| Marketing-authorisation holder | Various companies |
| More information | Azithromycin-containing medicinal products for systemic use |
| Name of medicine | Pradaxa |
|---|---|
| Marketing-authorisation holder | Boehringer Ingelheim International GmbH |
| More information | Pradaxa: Pending EC decision |