Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 9-12 December 2019
NewsHumanData on medicines
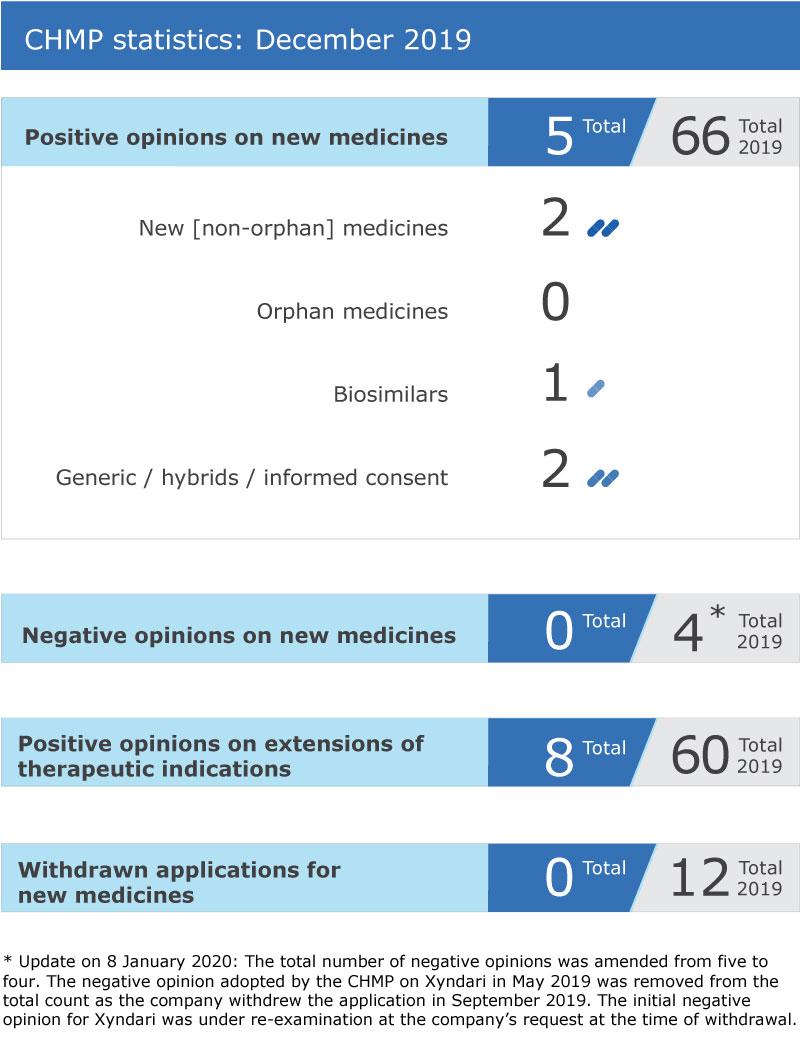
EMA’s human medicines committee (CHMP) recommended five medicines for approval at its December 2019 meeting.
The Committee recommended granting a marketing authorisation for Beovu (brolucizumab) for the treatment of neovascular (‘wet’) age-related macular degeneration, a disease that affects the central part of the retina at the back of the eye and causes loss of ‘straight-ahead’ vision.
The CHMP adopted a positive opinion for Recarbrio (imipenem / cilastatin / relebactam), for the treatment of infections due to aerobic Gram-negative organisms in adults with limited treatment options.
The biosimilar medicine Amsparity (adalimumab) received a positive opinion for the treatment of certain inflammatory and autoimmune disorders.
The CHMP recommended granting marketing authorisations for two generic medicines: Azacitidine Accord (azacitidine), for the treatment of myelodysplastic syndromes, chronic myelomonocytic leukaemia and acute myeloid leukaemia, diseases in which the body produces large numbers of abnormal blood cells; and Dexmedetomidine Accord (dexmedetomidine), for the induction of light to moderate sedation of adults in an intensive care unit.
The Committee recommended extensions of indication for Akynzeo, Cyramza, Darzalex, Dificlir, Erleada, Sirturo, Stelara and Vyndaqel.
The agenda of the December meeting is published on EMA's website. Minutes of the November 2019 CHMP meeting will be published in the coming weeks.
Key figures from the December 2019 CHMP meeting are represented in the graphic below.
| Name of medicine | Beovu |
| INN | brolucizumab |
| Marketing-authorisation applicant | Novartis Europharm Limited |
| Therapeutic indication | Treatment of neovascular (wet) age-related macular degeneration (AMD) |
| More information | Beovu: Pending EC decision |
| Name of medicine | Recarbrio |
| INN | imipenem / cilastatin / relebactam |
| Marketing-authorisation applicant | Merck Sharp & Dohme B.V. |
| Therapeutic indication | Treatment of infections due to aerobic Gram-negative organisms in adults with limited treatment options |
| More information | Recarbrio: Pending EC decision |
| Name of medicine | Amsparity |
| International non-proprietary name (INN) | adalimumab |
| Therapeutic indication | Treatment of certain inflammatory and autoimmune disorders |
| Marketing-authorisation applicant | Pfizer Europe MA EEIG |
| More information | Amsparity: Pending EC decision |
| Name of medicine | Azacitidine Accord |
| INN | azacitidine |
| Marketing-authorisation applicant | Accord Healthcare S.L.U. |
| Therapeutic indication | Treatment of myelodysplastic syndromes, chronic myelomonocytic leukaemia and acute myeloid leukaemia |
| More information | Azacitidine Accord: Pending EC decision |
| Name of medicine | Dexmedetomidine Accord |
| INN | dexmedetomidine |
| Marketing-authorisation applicant | Accord Healthcare S.L.U. |
| Therapeutic indication | Induction of light to moderate sedation of adults in intensive care unit |
| More information | Dexmedetomidine Accord: Pending EC decision |
| Name of medicine | Akynzeo |
| INN | fosnetupitant / palonosetron |
| Marketing-authorisation holder | Helsinn Birex Pharmaceuticals Limited |
| More information | Akynzeo: Pending EC decision |
| Name of medicine | Cyramza |
| INN | ramucirumab |
| Marketing-authorisation holder | Eli Lilly Nederland B.V. |
| More information | Cyramza: Pending EC decision |
| Name of medicine | Darzalex |
| INN | daratumumab |
| Marketing-authorisation holder | Janssen-Cilag International NV |
| More information | Darzalex: Pending EC decision |
| Name of medicine | Dificlir |
| INN | fidaxomicin |
| Marketing-authorisation holder | Astellas Pharma Europe B.V. |
| More information | Dificlir: Pending EC decision |
| Name of medicine | Erleada |
| INN | apalutamide |
| Marketing-authorisation holder | Janssen-Cilag International N.V. |
| More information | Erleada: Pending EC decision |
| Name of medicine | Sirturo |
| INN | bedaquiline |
| Marketing-authorisation holder | Janssen-Cilag International NV |
| More information | Sirturo: Pending EC decision |
| Name of medicine | Stelara |
| INN | ustekinumab |
| Marketing-authorisation holder | Janssen-Cilag International NV |
| More information | Stelara: Pending EC decision |
| Name of medicine | Vyndaqel |
| INN | tafamidis |
| Marketing-authorisation holder | Pfizer Europe MA EEIG |
| More information | Vyndaqel: Pending EC decision |