Veterinary big data
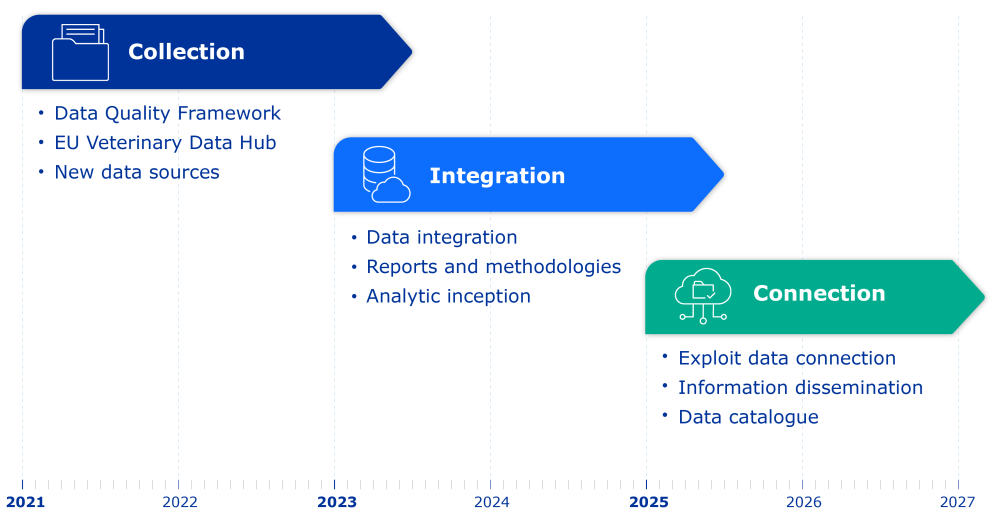
The European Medicines Agency (EMA) and Heads of Medicines Agencies (HMA) are jointly running a multi-year veterinary big data initiative, to help the European medicines regulatory network move towards a more data-driven culture in the regulation of veterinary medicines in the European Union (EU).
VeterinaryInnovation