Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 11-14 December 2023
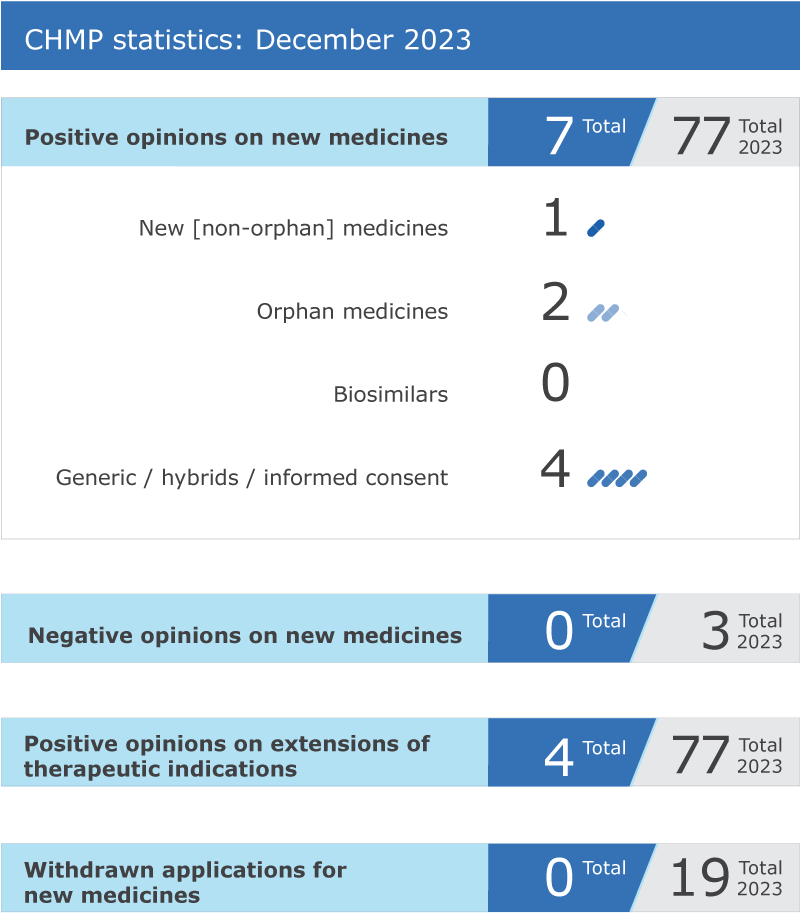
EMA’s human medicines committee (CHMP) recommended seven medicines for approval at its December 2023 meeting.
NewsHumanMedicinesReferrals
EMA’s human medicines committee (CHMP) recommended seven medicines for approval at its December 2023 meeting.
The committee recommended granting a conditional marketing authorisation for Casgevy* (exagamglogene autotemcel), an advanced therapy medicinal product (ATMP) for the treatment of transfusion dependent beta-thalassemia and severe sickle cell disease, two inherited rare diseases caused by genetic mutations that affect the production or function of haemoglobin, the protein found in red blood cells that carries oxygen around the body. This is the first medicine using CRISPR/Cas9, a novel gene-editing technology. Casgevy was supported through EMA's priority medicines (PRIME) scheme, which provides early and enhanced scientific and regulatory support for medicines that have a particular potential to address patients' unmet medical needs. See more details in the news announcement in the grid below.
Skyclarys* (omaveloxolone) received a positive opinion from the CHMP for the treatment of Friedreich’s ataxia, an inherited disease causing a range of symptoms that worsen over time, including difficulty walking, inability to co-ordinate movements, muscle weakness, speech problems, damage to the heart muscle and diabetes.
The committee adopted a positive opinion for Velsipity (etrasimod), for the treatment of patients with moderate to severe ulcerative colitis, an inflammation of the large intestine causing ulceration and bleeding.
Four generic medicines received a positive opinion from the committee:
Following a re-examination, the CHMP has confirmed its initial recommendation to not renew the conditional marketing authorisation for Blenrep* (belantamab mafodotin), a medicine used to treat multiple myeloma (a cancer of the bone marrow).
For more information, see the public health communication in the grid below.
The committee recommended extensions of indication for four medicines that are already authorised in the EU: HyQvia, Metalyse, VeraSeal and Zinplava.
The CHMP adopted positive opinions for two medicines:
These two medicines were submitted under a regulatory procedure known as EU-Medicines for all (EU-M4All) that enables EMA to support global regulatory capacity building and contribute to the protection and promotion of public health beyond the EU.
EMA has recommended the suspension of the marketing authorisations of a number of generic medicines tested by Synapse Labs Pvt. Ltd, a contract research organisation (CRO) located in Pune, India. The recommendation follows a good clinical practice (GCP) inspection which showed irregularities in study data and inadequacies in study documentation and in the computer systems and procedures to appropriately manage study data. A list of the medicines concerned is available on the EMA website.
For more information, see the public health communication in the grid below.
The agenda of the December 2023 CHMP meeting is published on EMA's website. Minutes of the meeting will be published in the coming weeks.
Key figures from the December 2023 CHMP meeting are represented in the graphic below
*This product was designated as an orphan medicine during its development. Orphan designations are reviewed by EMA's Committee for Orphan Medicinal Products (COMP) at the time of approval to determine whether the information available to date allows maintaining the medicine’s orphan status and granting the medicine ten years of market exclusivity.
exagamglogene autotemcel
Vertex Pharmaceuticals (Ireland) Limited
Treatment of transfusion-dependent β-thalassemia and sickle cell disease
omaveloxolone
Reata Ireland Limited
Treatment of Friedreich’s ataxia
etrasimod
Pfizer Europe MA EEIG
Treatment of patients with moderately to severely active ulcerative colitis (UC)
dabigatran etexilate
Viatris Limited
Prevention of venous thromboembolic events
ibuprofen
Gen.Orph
Treatment of a haemodynamically significant patent ductus arteriosus in preterm newborn infants less than 34 weeks of gestational age
eribulin
YES Pharmaceutical Development Services GmbH
Treatment of breast cancer and liposarcoma
pomalidomide
Viatris Limited
Treatment of adult patients with relapsed and refractory multiple myeloma (MM) in combination with dexamethasone
human normal immunoglobulin
Baxalta Innovations GmbH
Tenecteplase
Boehringer Ingelheim International GmbH
human fibrinogen / human thrombin
Instituto Grifols, S.A.
bezlotoxumab
Merck Sharp & Dohme B.V.
belantamab mafodotin
GlaxoSmithKline (Ireland) Limited
arpraziquantel
Merck Europe B.V.
Treatment of schistosomiasis in children
fexinidazole
Sanofi Winthrop Industrie
Various companies