Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 12-15 November 2018
NewsHumanMedicines
EMA’s human medicines committee (CHMP) recommended four medicines for approval, including a medicine for use in countries outside the European Union, at its November 2018 meeting.
The CHMP adopted a positive opinion for Fexinidazole Winthrop (fexinidazole), the first oral-only medicine (tablets) for the treatment of human African trypanosomiasis, commonly known as sleeping sickness, due to Trypanosoma brucei gambiense. This is the tenth medicine recommended by EMA under Article 58, a mechanism that allows the CHMP to assess and give opinions on medicines for use outside the European Union. For more information, please see the press release in the grid below.
Erleada (apalutamide) received a positive opinion for the treatment of non-metastatic castration resistant prostate cancer.
The CHMP recommended granting a marketing authorisation for Macimorelin Aeterna Zentaris (macimorelin), for the diagnosis of growth hormone deficiency in adults.
The generic medicine Silodosin Recordati (silodosin) received a positive opinion from the CHMP for the treatment of the signs and symptoms of benign prostatic hyperplasia.
Four recommendations on extensions of therapeutic indication
The Committee recommended extensions of indication for Kisqali, Mabthera, Orkambi and Ravicti.
Positive recommendations on extension of therapeutic indication following re-examination
The Committee recommended an extension of therapeutic indication for Blincyto (blinatumomab) in patients with residual cancer cells in the body after previous treatment, after re-examining its negative opinion for this medicine adopted in July 2018.
The CHMP also adopted a positive opinion for the use of Opdivo (nivolumab) and Yervoy (ipilimumab) in combination to treat renal cell carcinoma (kidney cancer), after re-examining its negative opinion adopted in July 2018.
For more information on these positive opinions following re-examination please see the question-and-answer documents in the grid below.
Outcome of review on quinolone and fluoroquinolone antibiotics
The CHMP recommended suspending some quinolone and fluoroquinolone antibiotics and introducing changes including restrictions on the use of all others following a review of disabling and potentially permanent side effects reported with these medicines. The review incorporated the views of patients, healthcare professionals and academics presented at EMA’s public hearing on these medicines in June 2018. For more information, please see the public health recommendation in the grid below.
Withdrawal of extension of indication application
The application to extend the use of Tecentriq (atezolizumab) to treat kidney cancer was withdrawn. A question-and-answer document on this withdrawal is available in the grid below.
Agenda and minutes
The agenda of the November 2018 meeting is published on EMA's website. Minutes of the October 2018 CHMP meeting will be published in the coming weeks.
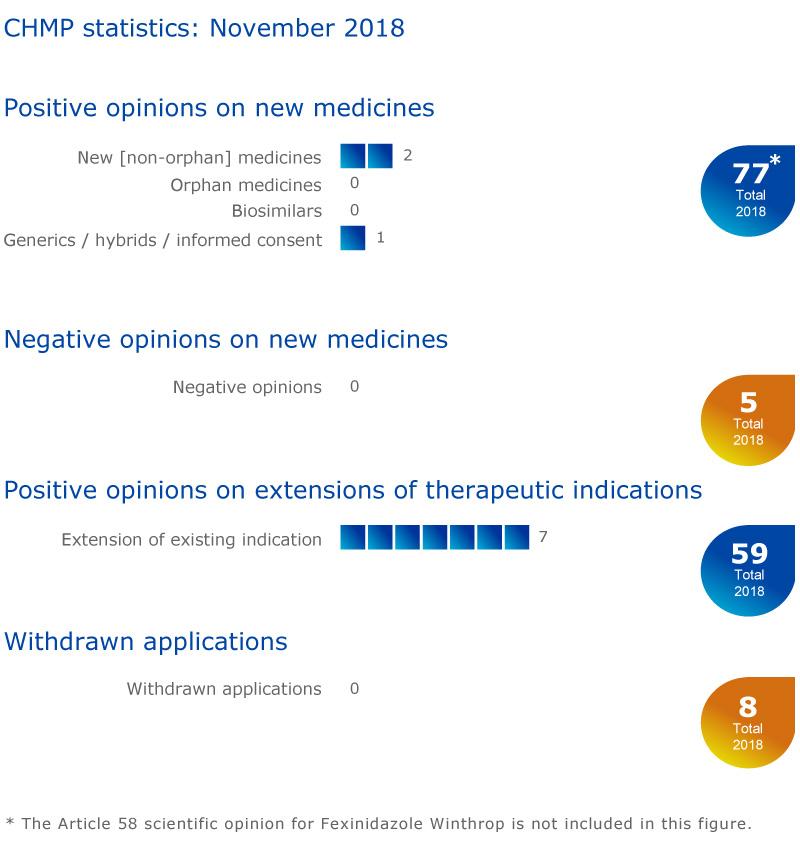
CHMP statistics
Key figures from the November 2018 CHMP meeting are represented in the graphic below.
| Name of medicine | Erleada |
|---|---|
| INN | apalutamide |
| Marketing-authorisation applicant | Janssen-Cilag International N.V. |
| Therapeutic indication | Treatment of non-metastatic castration resistant prostate cancer |
| Name of medicine | Macimorelin Aeterna Zentaris |
|---|---|
| INN | macimorelin |
| Marketing-authorisation applicant | Aeterna Zentaris GmbH |
| Therapeutic indication | Diagnosis of growth hormone deficiency in adults |
| Name of medicine | Silodosin Recordati |
|---|---|
| INN | silodosin |
| Marketing-authorisation applicant | Recordati Ireland Ltd |
| Therapeutic indication | Treatment of the signs and symptoms of benign prostatic hyperplasia |
| Name of medicine | Fexinidazole Winthrop |
|---|---|
| INN | fexinidazole |
| Opinion holder | sanofi-aventis groupe |
| Therapeutic indication | Treatment of human African trypanosomiasis (HAT) due to Trypanosoma brucei gambiense |
| More information | Press release: CHMP recommends first oral-only treatment for sleeping sickness |
| Name of medicine | Kisqali |
|---|---|
| INN | ribociclib |
| Marketing-authorisation applicant |
Novartis Europharm Limited |
| Name of medicine | Mabthera |
|---|---|
| INN | rituximab |
| Marketing-authorisation applicant | Roche Registration GmbH |
| Name of medicine | Orkambi |
|---|---|
| INN | lumacaftor / ivacaftor |
| Marketing-authorisation applicant | Vertex Pharmaceuticals (Europe) Ltd |
| Name of medicine | Ravicti |
|---|---|
| INN | glycerol phenylbutyrate |
| Marketing-authorisation applicant | Horizon Pharma Ireland Limited |
| Name of medicine | Blincyto |
|---|---|
| INN | blinatumomab |
| Marketing-authorisation applicant | Amgen Europe B.V. |
| Name of medicine | Opdivo |
|---|---|
| INN | nivolumab |
| Marketing-authorisation applicant | Bristol-Myers Squibb Pharma EEIG |
| Name of medicine | Yervoy |
|---|---|
| INN | ipilimumab |
| Marketing-authorisation applicant | Bristol-Myers Squibb Pharma EEIG |
| Name of medicine | Quinolone- and fluoroquinolone-containing medicinal products |
|---|---|
| INN | nalidixic acid, pipemidic acid, cinoxacin, enoxacin, pefloxacin, lomefloxacin, ciprofloxacin, levofloxacin, ofloxacin, moxifloxacin, norfloxacin, prulifloxacin, rufloxacin, flumequin |
| More information | Disabling and potentially permanent side effects lead to suspension or restrictions of quinolone and fluoroquinolone antibiotics |
| Name of medicine | Diclofenac sodium spray gel 4% |
|---|---|
| INN | diclofenac |
| Name of medicine | Diotop capsules |
|---|---|
| INN | diclofenac / omeprazole |
| Name of medicine | Tecentriq |
|---|---|
| INN | atezolizumab |
| Marketing-authorisation applicant | Roche Registration GmbH |