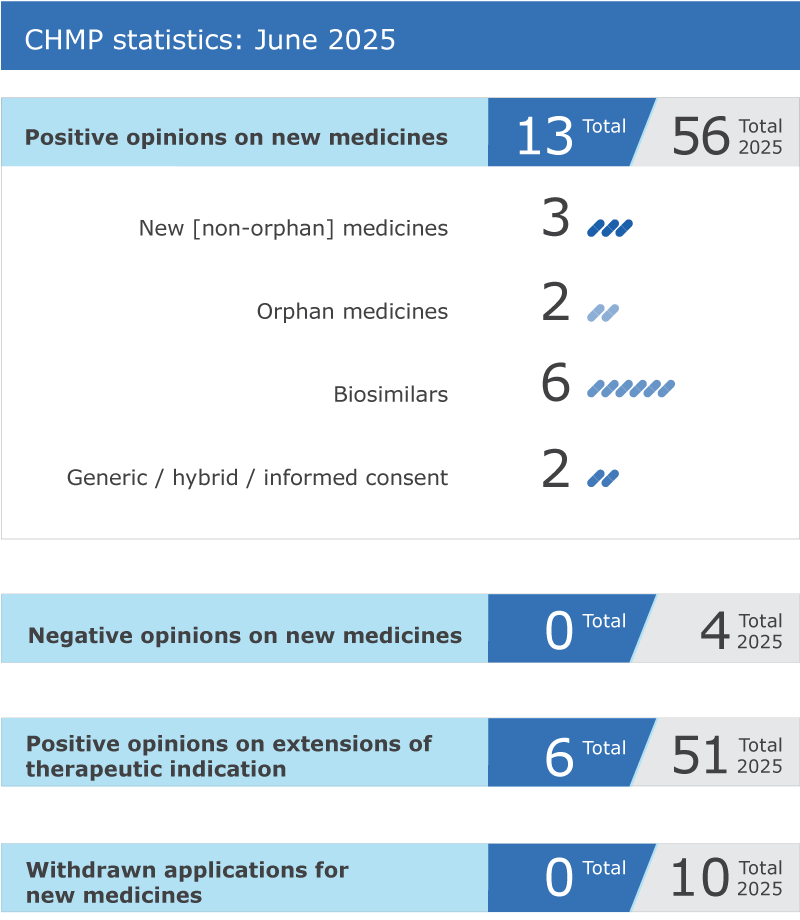
Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 16-19 June 2025
13 new medicines recommended for approval; another six medicines recommended for extension of their therapeutic indications
NewsHumanMedicinesMedicines for use outside the EUReferrals